ONE MORNING AS SHE DRESSED FOR WORK in July 2017, Tiffany Fagnani’s eye began to twitch. Then her arm began to twitch too. Fagnani, then 36, was working as a nurse in Hershey, Pennsylvania, and suspected she was about to have a stroke. “I thought, ‘I’m going to lose consciousness,’” she recalls. She knew what to do. While she still could, she called 911, unlocked her front door and grabbed her migraine medicine so the emergency responders would find it when they arrived. When they did, she was unconscious and unresponsive.
Fagnani had occasional migraines starting when she was 19 and, more recently, had lost weight and experienced vision problems. But she was busy: She managed care for her brother, who has cerebral palsy, and her parents had been living with her. In retrospect, she chides herself for not paying more attention to her symptoms. “Like a good nurse that I am,” she jokes, “I didn’t tell my doctor about the vision changes when I had a migraine check-in.”
A CT scan at the hospital revealed the latest episode wasn’t a stroke, but she did have a mass in her brain. Further imaging revealed another mass in one lung. She underwent a diagnostic bronchoscopy—a procedure that uses a narrow, glowing tube to examine the inside of a person’s lung. Biomarker testing on a sample of the lung mass revealed it was a tumor with a mutation in the EGFR gene. The diagnosis was both unwelcome and discouraging—stage IV non-small cell lung cancer that had spread to the brain. Stage IV disease is not curable, the oncologist told her, and he estimated she had six to 18 months to live. Fagnani was shocked and devastated. While she had smoked a little when she was younger, she had kicked the habit years before and was still young and in good health.

Tiffany Fagnani
Her first oncologist recommended that she consult with specialists, so Fagnani transferred her care to Fox Chase Cancer Center, about two hours away in Philadelphia. There she met with an oncologist who offered a different way forward. He treated Fagnani’s lung tumor with Tarceva (erlotinib), an oral drug that targets tumors containing certain EGFR genetic mutations, and with surgery to remove the brain tumor. She also met with a radiation oncologist to discuss radiation treatment that would target the lingering cancer cells and keep the cancer at bay.
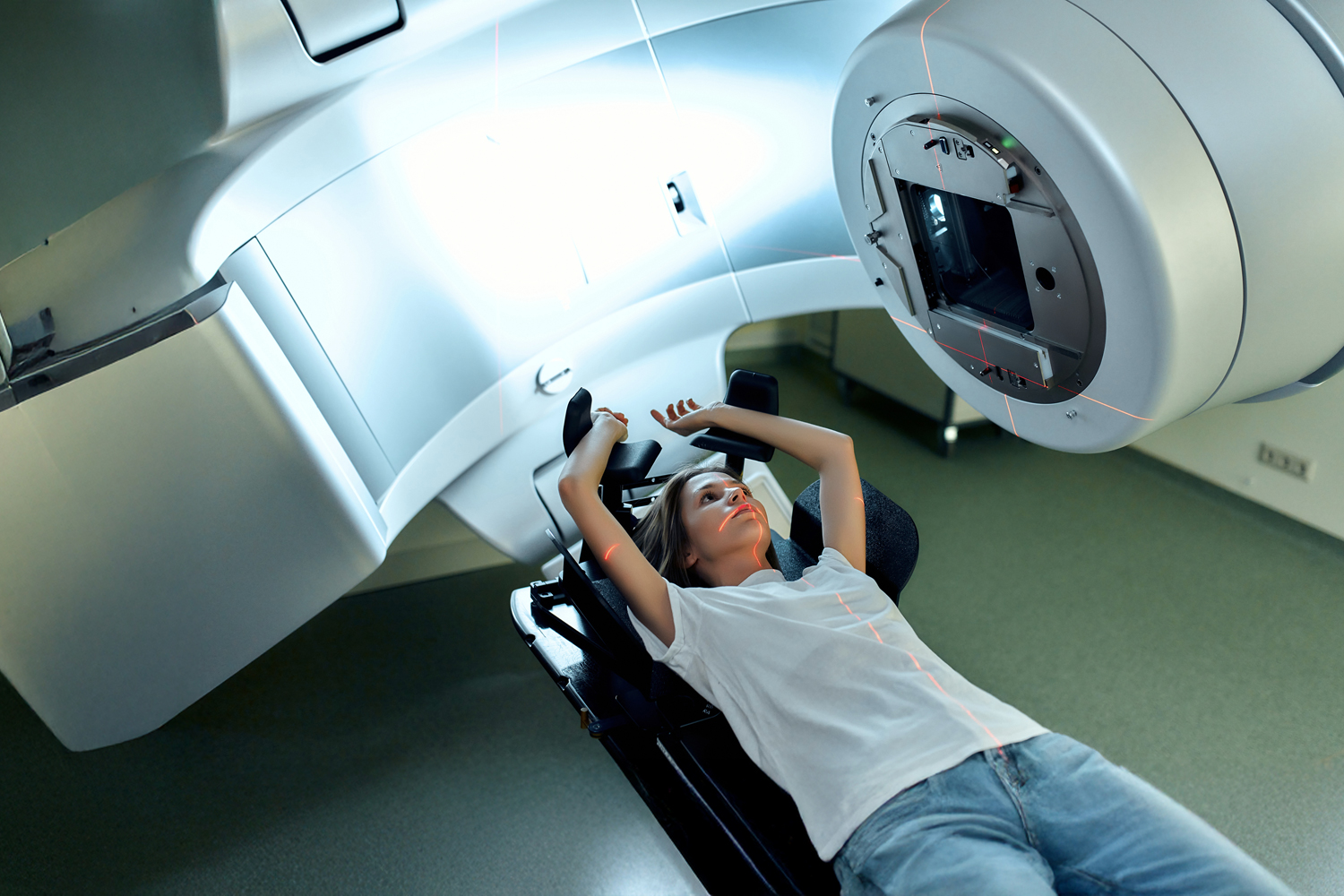
Radiation has been a mainstay of cancer treatment for more than a century, but over time, as researchers have learned more about how high-energy beams interact with living tissue, the application and efficacy of radiation have improved dramatically. Until the 1990s, someone like Fagnani would have received whole brain radiation treatment (WBRT), which, as the name suggests, applies radiation to the entire brain over several weeks. But whole brain therapy is a blunt instrument that can affect healthy tissue as well as the tumor. WBRT carries a formidable risk of side effects, including cognitive decline, but it is still used to treat people with multiple metastases to the brain.
Stereotactic body radiation therapy (SBRT) uses multiple beams of radiation, applied at different angles, that intersect at the area of the tumor. The idea is to deliver a large amount of radiation to the cancerous cells and a minimal amount to surrounding heathy cells. Because Fagnani had only one brain tumor, her radiation oncologist recommended she undergo stereotactic radiotherapy. The procedure was carried out by a robotic arm that changed position and angle throughout the session.
She only required three sessions. “I went on a Monday, Wednesday, Friday, and that was it,” she says.
Shorter, More Intense Radiation
SBRT is a type of precise hypofractionated radiation therapy. “Fractionation” refers to how the total radiation dose is divided into individual treatments, and hypofractionated or ultrahypofractionated schedules deliver larger doses of radiation, or fractions, in fewer visits. These shorter courses have become standard of care for many types of cancer. Although recommended treatment schedules vary by patient and by type and stage of cancer, the field has been converging on the shortest, safest possible treatment schedule backed up by evidence. Over the past 20 years, a raft of rigorous clinical trials has shown that, for many types of cancer, intense regimens of a few treatments, given over just a few days, work as effectively in targeting cancerous cells, and with a lower risk of side effects, as the 40-plus-session treatments that once served as the standard of care for many cancers. With these advances, some patients may only require a single treatment.
That means fewer visits to the hospital and lower costs. “Patients don’t like to come in any more than they have to; they don’t like being at the doctor’s,” says radiation oncologist Drew Moghanaki, chief of thoracic radiation oncology at UCLA Health Jonsson Comprehensive Cancer Center in Los Angeles, who has helped run clinical trials assessing the efficacy of shorter radiation schedules for people with lung cancer. When Moghanaki sees patients, he says, the goal is to deliver the most effective treatment in the shortest fractionation schedule possible. “We don’t treat anyone more than a week for a lot of cancers,” he says.
Cancer patients who are Black or treated at community health centers are less likely to get newer, shorter courses of treatment.
Tiffany Fagnani of Mechanicsburg, Pennsylvania, says she feels fortunate to have been close enough to a major cancer center in Philadelphia to receive fewer, but more intense, radiation treatments for her lung cancer. Despite the large body of evidence supporting short treatment courses, not all patients with cancer are given the option.
In a cohort study of more than 300,000 people in the United States with localized prostate cancer published Oct. 5, 2023, in JAMA Oncology, radiation oncologist Daniel Spratt, chair of radiation oncology at University Hospitals Seidman Cancer Center and Case Western Reserve University in Cleveland, and his collaborators found that in 2020, about 63% of patients received hypofractionated or ultrahypofractionated radiation schedules. That’s good news: The majority of patients received shorter radiation schedules. But there’s more work to be done. The rest of the patients, about 37%, received up to nine weeks of daily radiation treatments, even though multiple clinical trials have shown that shorter schedules are just as effective. According to the study, people who were Black or were treated at community health centers—rather than at major cancer centers or academic hospitals—were less likely to be treated with hypofractionated courses.
“If I was told, I need you to go [get radiation treatment] 40 times in a row and fit this into your life, for me, I don’t know how I would do it as a busy physician,” Spratt says, adding that doctors who trained in the 1990s, when radiation still meant 40 or more treatments, may be less comfortable recommending shorter courses.
“There is this residual concern for side effects, but there’s a mountain of evidence that shows these short courses are not more dangerous,” says radiation oncologist Drew Moghanaki of UCLA Health Jonsson Comprehensive Cancer Center in Los Angeles about the risks of shorter courses of treatment. Moghanaki adds that only institutions with advanced equipment and experts experienced in using the new technology should offer it to patients.
Finances might be an incentive to recommend longer courses since more visits means more income, but that incentive may soon disappear: Congress is currently considering a bill that would change Medicare so every radiation therapy course for cancer, no matter how many treatments it includes, would be reimbursed at the same rate.
Moghanaki says people newly diagnosed with cancer should ask about their options for short-course radiation treatments, especially if they’re initially offered a long course, and consider seeking a second opinion if the long course is the only option presented. “Patients are always trying to find out, is my doctor good enough? I recommend that if you can find a doctor who will deliver treatment in five treatments or less, you know that’s a good surrogate for them working at a pretty advanced center,” he says.
In recent years, large meta-analyses have found that hypofractionated schedules are as safe and effective as conventional, longer schedules for people with breast, lung, prostate and other cancers where it is now standard treatment. Not all patients receive these short schedules. For some cancers, like some head and neck cancers, the evidence is not conclusive for recommending it as standard of care. But even in cases where the evidence overwhelmingly supports short courses for efficacy and safety, not all patients are given the option, partly because they lack access to high-quality care, says radiation oncologist Daniel Spratt, chair of radiation oncology at University Hospitals Seidman Cancer Center and Case Western Reserve University in Cleveland.
Moghanaki wants to see that change. “I’ve learned that in cases where the evidence supports shorter courses of radiation for lung cancer, such as three to five treatments, not all patients are given the option,” he says. The evidence is there, he says, and now it’s a matter of changing the system to ensure that these shorter radiation schedules are available at all providers.
A Long-standing Cancer Treatment
In the late 1960s, scientists developed computed tomography (CT) scans, which compile image “slices” of an area to form a three-dimensional image. By the 1990s, radiologists regularly used CT scans to better guide treatments, and computers could help interpret the images. “You started to get the software to be able to say, ‘OK, I can look at a slice from a CT scan and see the prostate,’” says Spratt. From there, researchers developed tools that used four beams—two from the sides and one each from the front and back—to irradiate a cube-shaped area that included the prostate. By the 2000s, most cancer centers were using intensity-modulated radiation therapy (IMRT), which customizes radiation to deliver precise doses to a malignant tumor or specific areas of the tumor, Spratt says. For prostate cancer patients, the new technology resulted in rectal bleeding in only 1% to 3% of patients.
The early 21st century brought more intense focus on finding ways to target the smallest possible volume with the highest possible dose of radiation. “You could give a higher dose of radiation because you’re largely, but not completely, staying off the normal tissues,” says Spratt.
Radiologists have studied shorter, more intense radiation schedules across all types of cancer. A person diagnosed with breast cancer 60 years ago who received six weeks of daily radiation would probably undergo treatment today for three or four weeks. A meta-analysis of patient outcomes among people diagnosed with breast cancer, published in the BMJ Sept. 11, 2024, found that the safety profile and quality of life for patients on hypofractionated radiation schedules was better than conventional schedules. Ultrahypofractionated schedules, which gave the full dose in five treatments over one week, were just as effective and safe. The study also reported that survival and recurrence outcomes were roughly the same in all three groups. The message was clear: Given the shorter treatment time and convenience for patients, shorter is preferred.
Explore Your Options
Fagnani’s treatment shows how a short course can be more convenient than multiple visits. The doses she received used not one but multiple X-ray beams, from different angles, “shaped” to fit the geometry and size of the tumor. Even though she had to drive two hours to Philadelphia and two hours back home for each session, Fagnani only had to make the trip three times—and then she was done.
Short-course radiation still comes with side effects. Fagnani says her doctors told her she was at risk for seizures, which she didn’t experience, and for extreme fatigue, which she did. Fatigue from radiation accumulates over time with more exposure and may not show up until after the treatment has finished. That was the case with Fagnani. “I’m not really sure if it was side effects from having a craniotomy [surgery to remove the brain tumor] the month prior or the [SBRT] or the targeted therapy, but the fatigue caught up with me,” she says. “And when I would get tired, that was it. I was done. I did sleep a lot.” While she still has fatigue, she says, the extreme tiredness she first experienced slowly subsided after a couple of months.
At her first follow-up, in November 2017, two months after her radiation treatment, Fagnani’s scans showed no signs of metastases. In December 2018, however, a scan revealed a new tumor in her lung. Fagnani was concerned because the length of time since her diagnosis was close to the survival estimate her first oncologist had offered, and surgery isn’t recommended for patients with metastatic disease. But she did have a promising new option: In January 2019, her oncologist at Fox Chase prescribed Tagrisso (osimertinib), which had been approved by the Food and Drug Administration in April 2018 as a first-line treatment for people with metastatic NSCLC. A follow-up genetic test showed that her tumor tested positive for the EGFR T790M mutation, which is targeted by Tagrisso.
In July 2020, Fagnani underwent a course of radiation to her right lung to address remaining cancer cells. She underwent a hypofractionated schedule—five sessions over two weeks. In addition to fatigue, this time she developed radiation pneumonitis, which is inflammation in the lungs associated with high doses of radiation. It took a few weeks to fully recover from the radiation’s effects, but she says she would choose the same treatment again if given the choice.
After her diagnosis and treatments with surgery, targeted therapies and radiation, Fagnani’s disease is now stable. It’s been more than seven years since her first doctor told her she had less than a year to live. She volunteers as an advocate and often tells patients to explore all their options because the treatments and technology are changing quickly. Fagnani has visited labs to look at tumor cells under the microscope and spoken at conferences about her experiences. She makes it a point to keep up with new developments. “I wish I was on the other end of the table,” she says, “but it is exciting.”
Cancer Today magazine is free to cancer patients, survivors and caregivers who live in the U.S. Subscribe here to receive four issues per year.