WHEN QUOC-DIEN TRINH, a surgical oncologist and co-director of the Dana-Farber Cancer Institute in Boston, performs prostate cancer surgery, he sits at a large console about 15 feet away from his patient. From there, he uses a handheld controller and foot pedals to maneuver a robot equipped with four arms positioned over his patient. One of the robot’s arms contains a camera that creates a highly magnified, three-dimensional view of the tissue that Trinh will navigate through to locate the prostate and remove it. The other three arms have the surgical tools he will use to cut, sew, clamp and cauterize. Trinh uses the instruments to make small incisions in the patient’s pelvis to insert the robot’s arms, and he pulls out the patient’s prostate through one of the incisions.
Performing surgery has traditionally required cutting into and opening up the body. In the 1980s, technological advances made it possible for surgeons to perform minimally invasive surgeries using tiny cameras and miniature surgical tools inserted through small incisions. Doctors hailed these surgeries—called laparoscopic procedures—as an improvement for patients because they resulted in smaller scars, less bleeding and pain, and shorter hospital stays.
The robotic assistant was designed to make minimally invasive surgeries even better. The Food and Drug Administration (FDA) cleared the first surgical robot, called the da Vinci Surgical System, for general minimally invasive surgeries in July 2000. The following year, the FDA cleared the robot for prostate removal (radical prostatectomy) to treat men with prostate cancer. In 2005, the robots were cleared for use in gynecologic cancer surgeries. In 2017, a similar robot, called the Senhance System, received FDA clearance. As more hospitals began buying these $2 million machines, surgeons began using robots for a wider range of cancer procedures. And after seeing advertisements touting these robotic assistants—one ad described them as “where Star Trek meets Dr. Oz”—many patients sought them out. Studies show that patient demand for robotic surgeries has helped fuel their widespread adoption. Yet as the FDA warned in 2019, it’s not clear whether using a robot provides cancer patients with better outcomes than more traditional procedures.
Mixed Outcomes for Cancer Surgery
The bar for FDA clearance of a medical device is not as high as that for approval of a drug. It can take up to 15 years for a new drug to wend its way from initial discovery to FDA approval. In between are laboratory studies and clinical trials in humans, and only a minority of drugs ever get approved. The evaluation of new surgical techniques follows a different path. In this area of medicine, says Pedro Ramirez, a gynecologic oncologist at the University of Texas MD Anderson Cancer Center in Houston, “some surgeons start using a new technique and see a benefit, and then it becomes more and more popular. Then it is evaluated in studies that directly compare it to other techniques.”
Ramirez has been part of this process. After the da Vinci robot received FDA clearance for gynecologic procedures in 2005, Ramirez began using it in 2007 to treat patients with early-stage cervical cancer who required a radical hysterectomy, which involves removal of the cervix, the top of the vagina, the uterus and the tissue that surrounds it. As more surgeons began using the robotic assistant, Ramirez and his colleagues launched a clinical trial in June 2008 that randomly assigned 631 women with early-stage cervical cancer who needed a radical hysterectomy to one of two groups: traditional open surgery or minimally invasive surgery, which was further divided into conventional or robotic laparoscopy.
In June 2017, nine years into the study, Ramirez received an alert from the study’s data and safety monitoring committee. The committee had seen indicators suggesting that one group of patients was not doing as well and recommended enrollment be stopped until further analyses could be done. Ramirez and his colleagues began to collect additional follow-up information and further analyzed their data. Their findings, published in the Nov. 15, 2018, New England Journal of Medicine, revealed that the 319 women who had minimally invasive surgery—whether laparoscopic or robotic—had a higher rate of cancer recurrence and a higher likelihood of dying from cervical cancer than the 312 women who had open surgery. The findings took Ramirez and his colleagues by surprise. “One speculation is that it may be an issue of contamination,” he says, with cancer cells getting left behind as the tools move the tumor from inside to outside the body. But there was no question of what needed to be done. Minimally invasive surgeries for cervical cancer were brought to a halt in Ramirez’s clinic.
Other researchers were conducting similar studies. In 2011, an international group of surgeons and other experts launched a randomized clinical trial to compare robotic laparoscopic surgery to conventional laparoscopic surgery for treating rectal cancer. They wanted to see if using the robotic assistant made it more likely a surgeon could complete the procedure without having to switch to an open surgery. The study, published in JAMA in 2017, included 471 patients and found that the two surgical methods had about the same conversion rate to open surgery. “Anecdotally, we had felt there was a big role for robotic surgery, so we wanted to see if it was better,” says study co-author Alessio Pigazzi, a colorectal surgeon at the University of California, Irvine. “But it was not the silver bullet we thought it might be.”
Forty surgeons took part in the study, strengthening its findings. “Surgical studies are hard to do,” Pigazzi explains. “When you are comparing a technique or tool, there are other factors that come into play. It’s not like comparing drug A to a placebo or drug A to drug B. You have to take into account the surgeon’s skills, and that can make a big difference. What our study showed is that the robot is a really good tool, but if you are a very good surgeon you will get good results” whether or not you use it.
In 2010, Joshua Waltonen, a surgical oncologist at Wake Forest University Health Sciences in Winston-Salem, North Carolina, launched a study of robotic surgery in patients with certain types of human papillomavirus-related mouth and throat cancer. In the 1970s and 1980s, these cancers were typically treated with open surgeries. But in the 1990s, treatment with radiation and chemotherapy, which resulted in fewer long-term side effects, became the norm. The combination of chemotherapy and radiation “has excellent cure rates,” says Waltonen, but it can cause long-term difficulty with swallowing. The study’s finding that using the robot resulted in the “same survival and recurrence rates as radiation and chemotherapy” has made surgery a treatment option again. “The decision comes down to which treatment has less long-term toxicity and allows for better quality of life for each patient.”
A Mainstay for Prostate Cancer Surgery
Robotic assistants have made great inroads in treating prostate cancer. Today a majority of men whose prostate cancer is treated with a prostatectomy will undergo robotic surgery. Yet a study published in January 2018 in the British Journal of Cancer found that men whose prostatectomy was performed with a surgeon using a robot did not report better overall quality of life—nor better sexual, urinary or bowel function—than men who had traditional minimally invasive or open surgery. “In terms of length of stay in the hospital, robotic is better than open surgery, and in terms of blood loss, it is better, and patients need less pain medication,” says Trinh. And even though these benefits are the same for traditional minimally invasive surgery, robotic surgery for prostate cancer is here to stay. “Robotic prostatectomy is now how we do prostate removal,” says Trinh. “All of the surgeons do [the procedure using] the robot and it is how new surgeons are trained.”
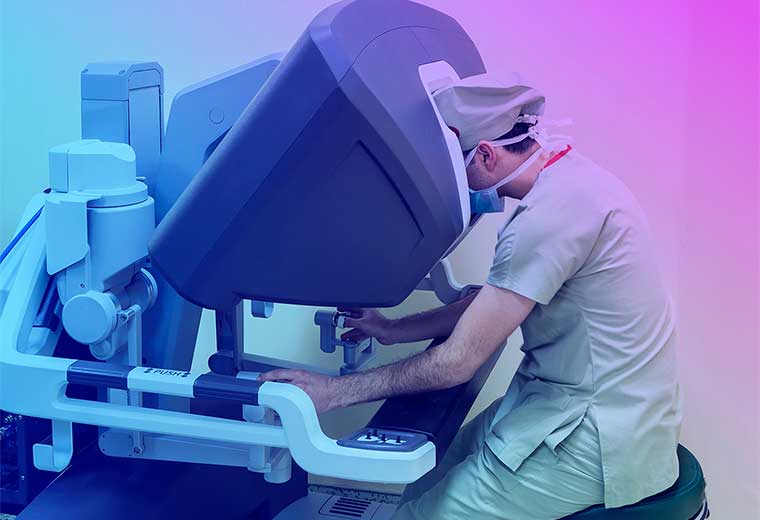
A surgeon performs an operation seated at the console of a surgical robot. The doctor, who is seated several feet away from the patient, controls the robot’s arms remotely. Photo by daphnusia / Shutterstock.com
The costs associated with robotic surgeries are higher than those for open surgeries. But that doesn’t mean patients are necessarily paying more out of pocket. A study of Trinh’s published in the Jan. 15, 2020, JAMA found that for certain gynecologic, colorectal, prostate and kidney cancer procedures, out-of-pocket costs were lower for patients who had robotic surgeries than for those who had open surgeries. Trinh’s study couldn’t answer why the cost was lower for robotic surgery. But he says one reason may be that hospitals are absorbing the cost they paid for the robot and the cost of the disposable equipment used in robotic procedures. (The robot’s instruments are typically not used more than 10 times.) “Dissemination happened on the back of a lot of patient demand due to direct-to-patient advertising,” Trinh says. “The message patients are sending is we want the latest or you won’t get [to provide] our care. So use is driven by patient demand, and if hospitals don’t adapt, they might lose patients.”
An FDA Warning
After a cancer drug has been approved, oncologists can use it off-label—meaning for cancers other than the ones for which it has been approved. Similarly, the FDA’s clearance of the da Vinci and Senhance robots made it possible for surgeons to use them for other surgeries where they thought patients might benefit. But in February 2019, after the FDA learned surgeons were using robotic assistants to perform mastectomies on women and men with breast cancer, the agency took action, warning the public that there were no data on the safety and effectiveness of using robots for these procedures. “We wanted [the public and providers] to know that robotically-assisted surgical devices have been evaluated by the FDA and cleared for use in certain types of surgical procedures, but not for mastectomy,” says Emma Spaulding, a press officer for the FDA.
But the FDA didn’t limit its warning to using robots for mastectomies. It also noted that the FDA did not have any information about whether robotic surgery improved any cancer patient’s outcomes. They cleared the robots based on data that showed how patients were doing 30 days after the procedure, not studies that looked at long-term outcomes.
The FDA’s statement led Kyle Sheetz, a surgeon at the University of Michigan in Ann Arbor, to co-author a viewpoint in the April 30, 2019, JAMA questioning whether more safeguards were needed to protect patients as robotic surgery becomes more widely adopted. “We’d been keeping an eye on trends around the country,” says Sheetz. “And as more operations were being done robotically, to some extent, it was a matter of time before someone began to question if there was sufficient evidence to justify the trends we were seeing.”
Sheetz finds the dramatic increase in the use of robotic surgery disconcerting, given the absence of data showing it is as good as or better than other surgical techniques. “We have been criticized as being anti-robot,” he says. “We aren’t. We are pro-evidence.” He also believes there needs to be better credentialing and training of doctors performing robotic surgeries. “There is a tendency to credential surgeons to use the technology, but not for a specific surgery,” he says. “And we don’t know if credentialing someone to use the robot in general and not for a specific surgery is putting patients at unnecessary risk.”
The Food and Drug Administration urges patients to ask specific questions about robotic surgery.
Before you have robotically-assisted surgery to prevent or treat cancer, the Food and Drug Administration advises that you:
- Be aware that the safety and effectiveness of robotically-assisted surgical devices for the prevention or treatment of cancer has not been established.
- Discuss the benefits, risks and alternatives of all treatment options with your health care provider.
- Ask the following questions before choosing your surgeon: What are your training and experience with robotically-assisted surgery? What have been the outcomes for your patients? How many robotically-assisted surgeries like mine have you performed? What are possible complications and how often do they happen?
If you had treatment with a robotically-assisted surgical device for cancer and experienced a complication, you can file a report through MedWatch, the FDA Safety Information and Adverse Event Reporting Program.
Patient Options
For cancer patients, Sheetz says, the FDA’s warning should serve as a reminder that in medical care, new doesn’t always mean better. He says it’s also important to think about what problem a new device is trying to solve. “For example, robotic surgery is marketed as having the benefit of a 3D camera, which gives better depth perception,” he says. “But there is no evidence that a 3D camera makes a surgeon a better surgeon.” That doesn’t mean robotic surgery isn’t right for certain patients, he adds. “If the surgeon has extensive experience doing it that way, it may be an excellent surgery for their cancer.”
The most important thing for patients to remember is that while tools matter, the surgeon matters more, says Trinh. “Patients need to know their surgeon’s experience,” he says. At the end of the day, “you are signing on to have a human being—not a robot—do the surgery.”
Cancer Today magazine is free to cancer patients, survivors and caregivers who live in the U.S. Subscribe here to receive four issues per year.





