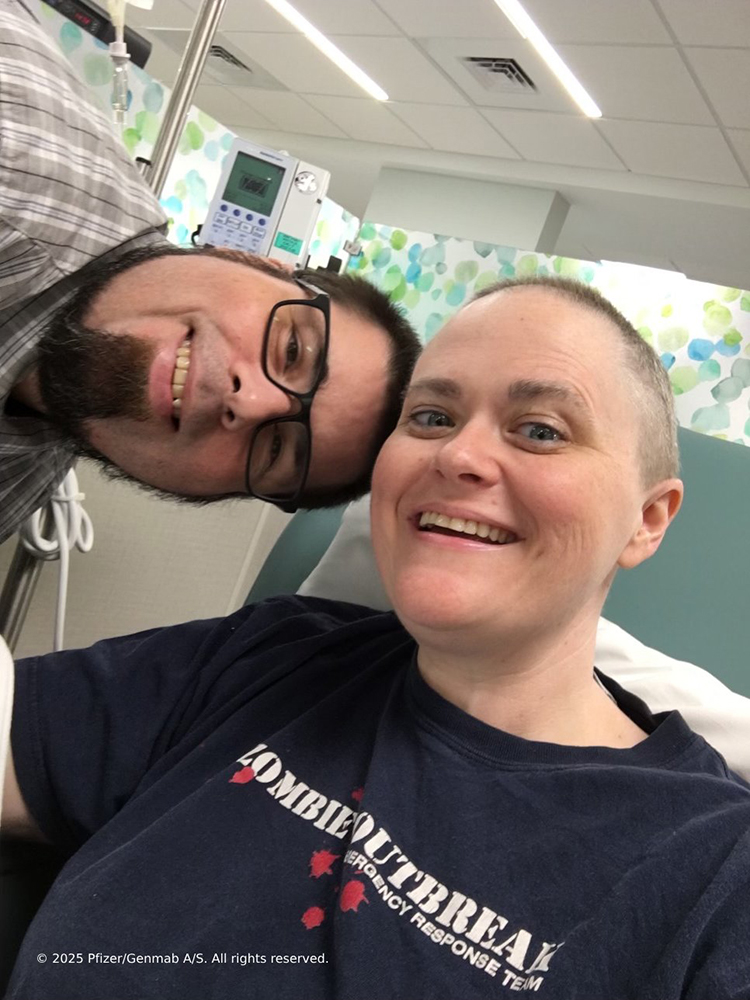
It never occurred to Courtney that the difficulties she was having with sleeping and breathing could be clues indicating a more serious health problem. When these symptoms became unbearable, she was rushed to the emergency room. Courtney, a 44-year-old pharmacy technician from Wilmington, North Carolina, was shocked that she was eventually diagnosed with stage III cervical cancer.
“It was all just kind of a whirlwind, like, ‘Oh my God. This is really happening,’” Courtney says. “Other than being screened for cervical cancer at my gynecologist, I knew hardly anything about it.”

© 2025 Pfizer/Genmab A/S. All rights reserved.
The American Cancer Society estimated there were 13,820 new cases of cervical cancer in 2024.i These numbers have improved since the mid-1970s due to advances in vaccination and screening practices, but despite this progress, an estimated 4,360 women were projected to die from the disease last year.i This underscores the need for more treatment options.
Following her initial diagnosis, Courtney began a treatment regimen, undergoing a hysterectomy and trying numerous therapies. When she progressed after first line treatment, she was diagnosed with recurrent cervical cancer. “That was really scary. It was a very hard Thanksgiving that year.”
When cervical cancer recurs or metastasizes, treatment options are limited, and the five-year relative survival rate is 19.4%.ii The outlook can feel bleak for patients and their loved ones. For Courtney, having open discussions with her health care providers helped her gain a full understanding of her treatment options.
Courtney recalls doing research on the internet and learning about TIVDAK® (tisotumab vedotin-tftv), an antibody-drug conjugate that targets tissue factor, which are proteins found on the surface of cervical cancer cells. The special protein, Tissue Factor, is also present on some normal cells. So Tivdak can still harm normal cells, which can cause side effects. “I wanted to learn everything about it. I was excited to try something else that could possibly work for me.”
After much consideration, Courtney and her medical team chose TIVDAK as her next treatment option, and she began receiving treatment with TIVDAK. TIVDAK is a prescription medicine used to treat adults with cervical cancer that has returned or has spread to other parts of the body, and who have received chemotherapy that did not work or is no longer working. In a clinical trial of 502 adult patients with cervical cancer that has returned or spread and who have received chemotherapy that did not or is no longer working, patients in the TIVDAK group had a median overall survival of 11.5 months compared with 9.5 months for those treated with chemotherapy.iii
© 2025 Pfizer/Genmab A/S. All rights reserved.
Eye problems are common with TIVDAK and can be severe. TIVDAK can cause changes to the surface of your eye that can lead to dry eyes, eye redness, eye irritation, corneal ulcers, blurred vision, and severe vision loss. Tell your healthcare provider if you develop new or worsening vision changes or eye problems during treatment. Serious side effects of TIVDAK may include eye problems, nerve problems (peripheral neuropathy), bleeding problems (hemorrhage), lung problems and severe skin reactions. These are not all the side effects of Tivdak. Please see below for more Important Safety Information and click here for the full Prescribing Information with Medication Guide.
“[Since starting TIVDAK], I feel hopeful,” Courtney says. “It was a really great option for me.” While these were Courtney’s experiences at the time that this article was published, TIVDAK will not work for everyone, and individual results may vary.
Having a support system was crucial for Courtney as she navigated treatment. In addition to feeling bolstered by her health care providers, husband, family and work colleagues, Courtney also found support in online patient and caregiver communities.
“It’s been amazing—the support that comes around you—sometimes from people you didn’t even expect. It’s been surprising and very encouraging. There are people working every day to help people just like me. I’m so thankful for that.”
If you or a loved one has previously treated recurrent or metastatic cervical cancer, speak with your health care provider about whether TIVDAK could be part of your treatment plan.
COM-US-TIV-0000331
PP-T1V-USA-0429
What is TIVDAK?
TIVDAK is a prescription medicine used to treat adults with cervical cancer:
- that has returned or has spread to other parts of the body, and
- who have received chemotherapy that did not work or is no longer working.
IMPORTANT SAFETY INFORMATION
What is the most important information I should know about TIVDAK?
Eye problems are common with TIVDAK and can be severe. TIVDAK can cause changes to the surface of your eye that can lead to dry eyes, eye redness, eye irritation, corneal ulcers, blurred vision, and severe vision loss. Tell your healthcare provider if you develop new or worsening vision changes or eye problems during treatment.
Your healthcare provider will send you to an eye specialist to check your eyes before you start treatment with TIVDAK, before each infusion for your first 9 infusions of TIVDAK, and as needed for any new or worsening signs or symptoms of eye problems. Your healthcare provider will ask if you have any signs or symptoms of eye problems before each infusion. You will be referred to an eye specialist for any new or worsening signs or symptoms of eye problems.
Your healthcare provider will prescribe 3 different types of eye drops before you start treatment with TIVDAK. Bring the eye drops with you to each infusion and use them as directed by your healthcare provider to reduce your risk of eye problems:
- Use 1 drop of steroid eye drops in each eye before each infusion and continue to use your eye drops 3 times a day for 3 days after each infusion
- Use vasoconstrictor eye drops right before each infusion
- Use lubricating eye drops throughout treatment and for 30 days after your last dose of TIVDAK
Do not wear contact lenses throughout your treatment with TIVDAK unless you are told to use them by your eye specialist.
What are the possible side effects of TIVDAK?
Serious side effects of TIVDAK may include:
Eye problems. See “What is the most important information I should know about TIVDAK?”
Nerve problems (peripheral neuropathy) are common with TIVDAK and can be serious. Tell your healthcare provider right away if you have new or worsening numbness or tingling in your hands or feet or muscle weakness.
Bleeding problems (hemorrhage) are common with TIVDAK and can be serious. Tell your healthcare provider or get medical help right away if you have signs or symptoms of bleeding during treatment with TIVDAK, including blood in your stools or black stools (looks like tar), blood in your urine, cough up or vomit blood, unusual vaginal bleeding, or any unusual or heavy bleeding.
Lung problems. TIVDAK may cause severe or life-threatening inflammation of the lungs that can lead to death. Tell your healthcare provider right away if you have new or worsening symptoms, including trouble breathing, shortness of breath, or cough.
Severe skin reactions. TIVDAK may cause severe or life-threatening skin reactions that can lead to death. Tell your healthcare provider or get medical help right away if you have signs or symptoms of a severe skin reaction during treatment with TIVDAK, including:
- Skin reactions that look like rings (target lesions)
- Rash or itching that continues to get worse
- Blistering or peeling of the skin
- Painful sores or ulcers in your mouth, nose, throat, or genital area
- Fever or flu-like symptoms
- Swollen lymph nodes
The most common side effects of TIVDAK include:
- Decreased red blood cell counts
- Numbness or tingling in your hands or feet
- Eye problems (conjunctival disorders)
- Nausea
- Tiredness
- Changes in liver function blood tests
- Nosebleed
- Hair loss (alopecia)
- Bleeding (hemorrhage)
Your healthcare provider may decrease your dose of TIVDAK, temporarily stop, or completely stop treatment if you have side effects.
What should I tell my healthcare provider before receiving TIVDAK?
Tell your healthcare provider about all your medical conditions, including if you:
- have a history of vision or eye problems
- have numbness or tingling in your hands or feet
- have bleeding problems
- have liver problems
- are pregnant or plan to become pregnant; TIVDAK can harm your unborn baby. Tell your healthcare provider right away if you become pregnant or think you may be pregnant during treatment with TIVDAK
- are breastfeeding (nursing) or plan to breastfeed. It is not known if TIVDAK passes into your breast milk. Do not breastfeed during treatment and for at least 3 weeks after the last dose of TIVDAK
Females who can become pregnant:- Your healthcare provider should do a pregnancy test before you start treatment with TIVDAK
- Use an effective method of birth control during your treatment and for at least 2 months after the last dose of TIVDAK
- Use effective birth control during treatment and for 4 months after the last dose of TIVDAK
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Taking TIVDAK with certain other medicines may cause side effects.
These are not all of the possible side effects of TIVDAK. Discuss side effects with your healthcare provider. You may report side effects to FDA at 1-800-FDA-1088.
Please see the full Prescribing Information with Medication Guide about TIVDAK including an IMPORTANT WARNING here.
Content sponsored and provided by Pfizer, Inc. and Genmab A/S. Courtney, the patient featured in this article, is receiving or has received TIVDAK. Courtney partnered with Pfizer, Inc. and Genmab A/S to share her experience with recurrent/metastatic cervical cancer and was compensated by Pfizer, Inc. and Genmab A/S to share her story. These were her experiences at the time she was interviewed. TIVDAK will not work for everyone. Individual results may vary.
© 2025 Pfizer/Genmab A/S. All rights reserved. TIVDAK is a trademark of Seagen Inc.
i American Cancer Society. (n.d.). Key statistics for cervical cancer. Retrieved September 6, 2024, from https://www.cancer.org/cancer/types/cervical-cancer/about/key-statistics.html.
ii SEER*Explorer: An interactive website for SEER cancer statistics [Internet]. Surveillance Research Program, National Cancer Institute; 2024 Apr 17. [updated: 2024 Nov 5; cited 2024 Dec 5]. Available from: https://seer.cancer.gov/statistics-network/explorer/. Data source(s): SEER Incidence Data, November 2023 Submission (1975-2021), SEER 22 registries (excluding Illinois and Massachusetts). Expected Survival Life Tables by Socio-Economic Standards.
iii Vergote, I., González-Martín, A., Fujiwara, K., Kalbacher, E., Bagaméri, A., Ghamande, S., Lee, J.-Y., Banerjee, S., Maluf, F. C., Lorusso, D., Yonemori, K., Van Nieuwenhuysen, E., Manso, L., Woelber, L., Westermann, A., Covens, A., Hasegawa, K., Kim, B.-G., Raimondo, M., … Slomovitz, B. M. (2024). Tisotumab Vedotin as second- or third-line therapy for recurrent cervical cancer. New England Journal of Medicine, 391(1), 44–55. https://doi.org/10.1056/nejmoa2313811
Cancer Today magazine is free to cancer patients, survivors and caregivers who live in the U.S. Subscribe here to receive four issues per year.
Disclaimer: Sponsored advertising content is written by the advertiser and does not necessarily reflect the views of Cancer Today or the American Association for Cancer Research (AACR). Cancer Today’s editorial staff was not involved in developing or creating the sponsored content. Content on the Cancer Today website is not intended to be a substitute for professional medical advice. For diagnosis or treatment of any medical condition or any questions you may have about a medical condition, please consult your physician or other qualified health care provider. Neither Cancer Today nor the AACR is liable for any loss or damage incurred as a result of reading or following the information or engaging with any of the advertising or sponsored advertising content on the Cancer Today website. The mention of any company, service, product or treatment does not constitute an endorsement by Cancer Today or the AACR.





