Editor’s Note: This story, written by Neha J. Pancholi, PhD, first appeared on Cancer Research Catalyst, the official blog of the American Association for Cancer Research (AACR). The AACR also publishes Cancer Today. You can read this and other stories on the AACR website.
DETECTING CANCER EARLY—when it is small and easier to treat—is key to saving lives. Routine screening tests like mammograms and colonoscopies check for cancer in healthy people and have drastically improved our ability to catch cancer before it turns deadly. But the unfortunate truth is that these screening tests aren’t enough.
Many of the deadliest cancers still don’t have any screening tests available, which means they often aren’t found until it’s too late.
And staying up to date with the cancer screenings that are available isn’t easy. A 45-year-old woman, for example, might need to have a mammogram, colonoscopy, Pap smear and skin check all in the same year—and that’s just if she has an average cancer risk. Depending on her family history and lifestyle, doctors might advise even more screenings for additional cancer types.
Each of these cancer screenings requires its own appointment with a different specialist, taking time to schedule, travel to and complete. On top of the immense time commitment, these tests might also lead to heightened anxiety and physical discomfort.
But what if there were an easier way? Routine blood tests already help doctors find signs of diabetes, heart disease, organ dysfunction and other medical conditions.
Could a blood test detect cancer too?
The answer, according to many researchers, is a resounding yes.
Known as “liquid biopsy,” this strategy differs from traditional tumor biopsies that rely on surgically extracting tissue from a patient who is suspected of having cancer. The advantages of liquid biopsy are that it is noninvasive (all you need from the patient is a blood sample), it could someday be performed as part of routine bloodwork even for patients with no signs of cancer, and it could even look for multiple types of cancers at once (unlike existing screening tests that look for cancer in a single organ).
Researchers are also exploring using other bodily fluids to test for cancer—looking for signs of cancer in breast milk or urine, for example.
How Does Liquid Biopsy Detect Cancer?
Like all cells in the body, cancer cells release molecules into the patient’s bloodstream. If researchers can identify molecules released specifically by cancer cells, they can develop a test that looks for these molecules in blood samples to find cancer earlier and more easily than is currently possible.
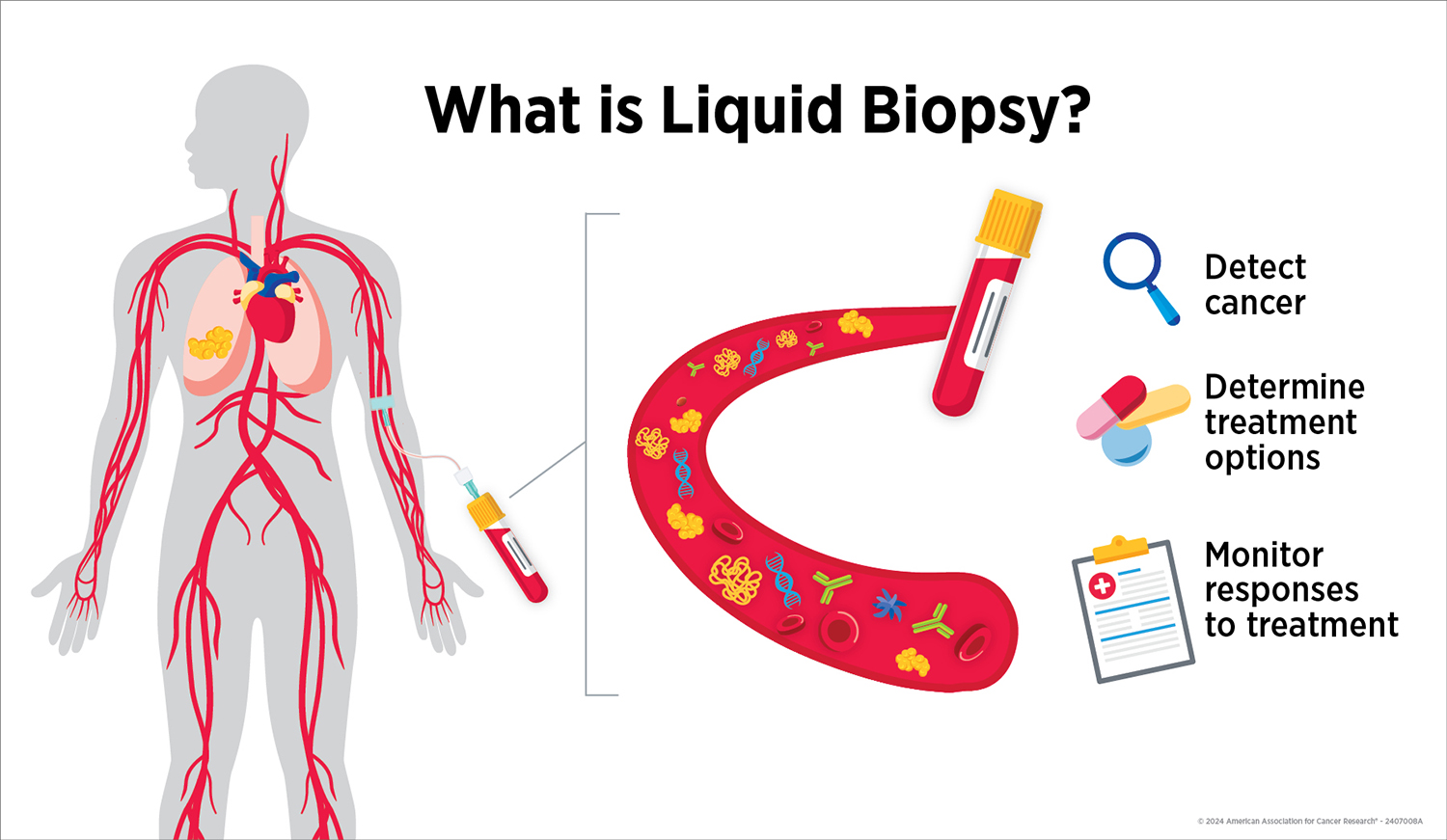
Researchers are developing liquid biopsies to look for signs of cancer in blood. In this illustration, a blood sample collected from a patient contains cells, DNA and other molecules released by their lung tumor, which may be used to detect, characterize and monitor their cancer.
Are Liquid Biopsy Tests FDA-approved for Early Cancer Detection?
A longstanding blood test to detect signs of prostate cancer has been approved by the U.S. Food and Drug Administration (FDA) since 1986, but this test (which looks for elevated levels of a protein called PSA) can be unreliable and its utility has been debated by experts.
In the intervening years, researchers have continued to develop and evaluate different liquid biopsies for cancer screening, and on July 29, 2024, the FDA approved Shield, a blood test that screens for colorectal cancer in people at least 45 years of age who have an average risk for the disease. The approval was based on clinical trial results that showed the test accurately detected 87.5% of nonmetastatic colorectal cancers and almost 90% of metastatic colorectal cancers.
Researchers are also working on newer tests that can detect multiple types of cancer from a single blood draw in people with no apparent signs of cancer. While some multicancer early detection (MCED) liquid biopsies are commercially available, none are currently approved by the FDA. Additional research is needed to improve their ability to detect cancers at early stages and to understand how impactful these tests would be at saving lives. Some experts worry that these tests could, in some cases, detect slow-growing tumors that would have been harmless if left alone, leading to unnecessary treatments that come with side effects, financial costs and stress. The benefits and risks of MCED liquid biopsies, therefore, need to be carefully weighed.
How Else Can These Tests Be Used?
Beyond early cancer detection, liquid biopsy can also be used to guide treatment in patients already diagnosed with cancer. Some liquid biopsy tests are FDA-approved to assess a patient’s eligibility for certain cancer treatments or to monitor how their cancer responds to treatment. These tests analyze molecules released by cancer cells into blood as a way to determine the tumor’s molecular characteristics and to assess tumor size before, during and after treatment. This information can help oncologists decide the next steps in disease management.
Cancer Today magazine is free to cancer patients, survivors and caregivers who live in the U.S. Subscribe here to receive four issues per year.





