Every week, the editors of Cancer Today magazine bring you the top news for cancer patients from around the internet. Stay up to date with the latest in cancer research and care by subscribing to our e-newsletter.
Do New Screening Recommendations for Mammogram Go Far Enough?
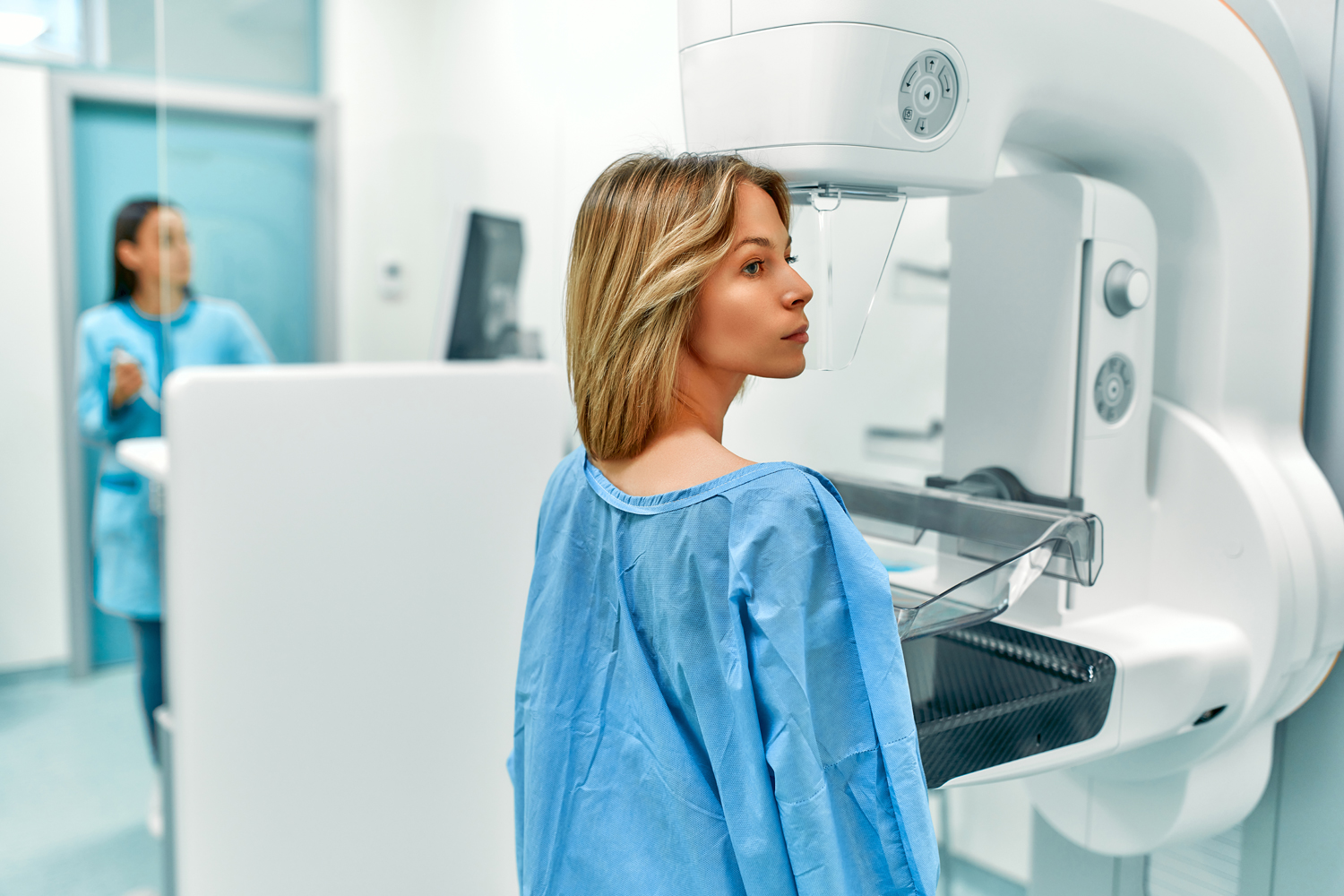
The U.S. Preventive Services Task Force (USPSTF) revised its breast cancer screening recommendations, suggesting mammography for women every other year from ages 40 to 74 years. These recommendations, published April 30 in JAMA, represent a change to the USPSTF’s guidance. Previously, the panel recommended biennial screening starting at age 50 and suggested individual choice in a person’s 40s. An editorial published the same day in JAMA Oncology criticized the every-other-year recommendation, noting that several professional health organizations recommend annual screening after 40. Critics also noted how the guidelines stopped short of recommending breast ultrasound or MRI in women with dense breasts if mammograms did not show troubling findings. A New York Times article said some doctors order ultrasound or MRI for women with dense breasts, given the increased risk of breast cancer and the potential difficulty in seeing lumps on mammograms due to dense tissue. The guidance suggests there isn’t enough evidence to recommend these tests at this time, which opens the possibility that insurance will not cover these extra screens as part of preventive care. “What that means for coverage is that there is no mandate to cover these specific screenings for women with dense breasts at zero-dollar cost-sharing,” said Robert Traynham, a spokesman for AHIP, the association that represents health insurance companies.
FDA to Provide Oversight of Lab Tests, Including Those That Help Direct Treatment for Cancer
The Food and Drug Administration (FDA) finalized a rule that will gradually phase in oversight of new tests developed by laboratories—although some experts said these efforts will be challenged in court. Makers of medical lab tests that have escaped government oversight will have about four years to show that their new offerings deliver accurate results. The goal is to ensure that new tests, including those that are used to direct cancer treatment, are safe for consumers. In a Washington Post article on the final rule, the FDA said it will grandfather those tests that are already on the market into approval to address concerns that the new rule “could lead to the widespread loss of access to beneficial” tests. Previous reporting from the Washington Post shared research by the nonprofit Friends of Cancer Research, which found substantial variability in diagnostic tests used to identify cancer patients who are most likely to benefit from immunotherapy. “In oncology, the stakes are high,” said Jeff Allen, president and chief executive of the organization. If the test results are not accurate, people could miss out on treatments that can help them or get treatments that won’t help, Allen says.
Understanding Secondary Cancer Risk Related to CAR T-cell Therapy
Since the Food and Drug Administration (FDA) now requires drug companies to include warnings about increased cancer risk on the drug packaging for CAR T-cell therapy, researchers and physicians are scrutinizing their own data from patients who have received CAR T-cell therapy to assess how big a risk there is—and to try to effectively communicate those risks to patients. An article published April 30 in Nature describes recent research efforts to help learn more about what is believed to be a relatively uncommon side effect of the treatment. As of March 25, the FDA received 33 reports of immune-cell cancers known as lymphomas out of about 30,000 people who had treatment, the article notes. More physicians are talking about the risk to people who are considering CAR T-cell therapy. Aric Hall, a hematologist at the University of Wisconsin–Madison, presents it as a real risk to patients, but a rare one—and explains the diagnosis poses a larger danger for patients. “For my late-stage myeloma patient, the main risk is that the CAR T doesn’t work, and they die of their myeloma,” Hall told Nature. He noted that as CAR T-cell therapy moves into a bigger pool of patients who aren’t desperately ill, the calculus could change.
Cancer Today magazine is free to cancer patients, survivors and caregivers who live in the U.S. Subscribe here to receive four issues per year.