Every week, the editors of Cancer Today magazine bring you the top news for cancer patients from around the internet. Stay up to date with the latest in cancer research and care by subscribing to our e-newsletter.
Compression and Cooling Therapy Ease Neuropathy in Hands and Feet
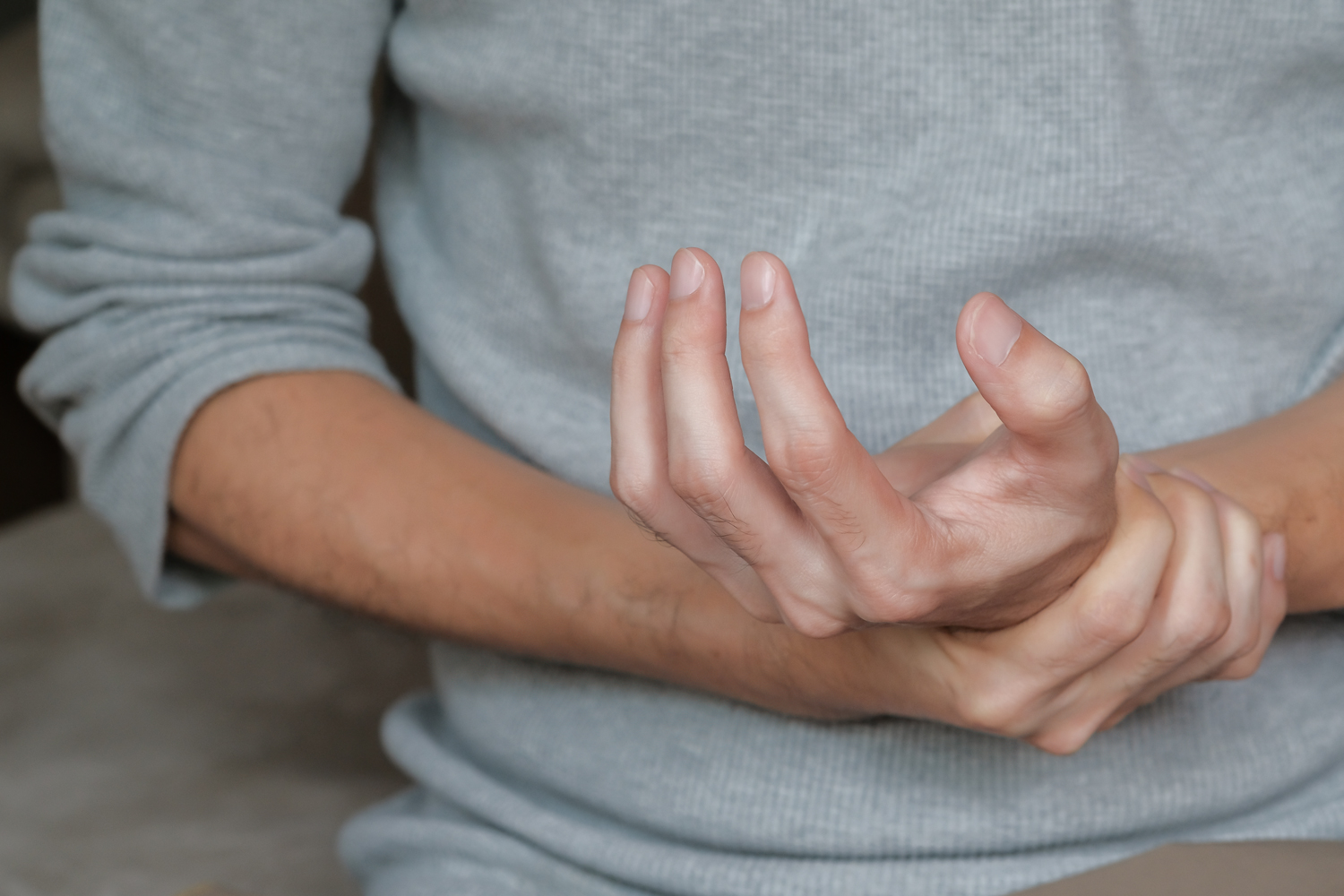
Chemotherapy-induced peripheral neuropathy, characterized by numbness, pain and tingling in the hands and feet, can linger long after chemotherapy treatment is completed. A study published in JAMA Oncology showed that ice or compression reduced the risk of developing grade 2 or higher peripheral neuropathy by nearly 50%. The analysis included 101 women with breast cancer who received taxane-based chemotherapy as part of their care. Each wore either a frozen glove or two tight-fitting surgical gloves on one hand, while wearing nothing on the other hand. The women in the study wore the gloves during chemotherapy treatment and for 30 minutes before and after the infusion, Medscape reported. In the study, cooling reduced the risk of developing peripheral neuropathy by 42%, and compression reduced it by 37%. The effects persisted for up to six to eight months after treatment.
Deadlines Encourage People to Return Colorectal Screening Tests
Deadlines keep projects moving forward, but a study published in the Lancet suggests a two-week deadline can also motivate people to follow through with colorectal cancer screening. In the study, almost 40,000 people who were part of Scotland’s nationwide screening program were divided into eight groups, each of which received mailed invitations to complete at-home colorectal cancer screening using a supplied fecal immunochemical test (FIT). Invitations were sent to groups with different instructions, including providing one-, two- or four-week deadlines or no deadline to return the test. Some groups also received a planning tool, which helped people identify concerns and provided tips for moving forward. In the study, the highest return rate was 68% for those with a two-week deadline without the planning tool. For comparison, 66% of those in the control group, whose invitation had no deadline or planning tool, completed the test. While representing a modest increase, the findings help to target communications to increase screening, the study authors noted. “We estimate that a 2% increase in FIT returns would mean an additional 39,000 people participating in a two-year Scottish Bowel Screening round, with approximately 23 colorectal cancer deaths being avoided as a result,” authors of the study explained in a Medpage Today article. “The implications of the findings for practice are that mailed invitations to FIT screening should consider including a deadline for FIT return—a two-week deadline might offer the best option in terms of impact and acceptability of the deadlines we tested.”
CAR T-Cell Therapy Shows Effectiveness in Brain Cancer
Results from a clinical trial published Jan. 5 in Nature Medicine suggest that CAR T-cell therapy, approved for treating certain blood cancers like leukemia and lymphoma, could offer some hope for treating a deadly brain tumor. Diffuse intrinsic pontine glioma (DIPG) affects mostly children and young adults, and those diagnosed with DIPG have a median life expectancy of about 10 months. As part of the clinical trial, 21 children and young adults with DIPG received CAR T-cell therapy, a treatment that removes a patient’s immune cells and genetically modifies them to better recognize and attack cancer cells. Participants received infusions of these genetically modified cells into the cerebrospinal fluid every two to four weeks. Participants in the study lived an average of about 20 months from their diagnosis—nearly double the prognosis. An article in NBC News describes how three patients are still alive more than three years after starting their treatment. One patient, Gavin Nielsen, who was diagnosed with DIPG when he was 2, is now six years old, NBC News reported. Still, investigators from the trial note that this study was designed to test the safety and feasibility of an treatment that is still considered experimental for this type of cancer. “When you’re dealing with experimental therapies, especially phase I trials, they’re designed to find what is a safe, tolerable dose. You obviously hope there’s a benefit, but it is impossible to quote any benefit, because you’re learning this in real time,” Nicholas Vitanza, a pediatric neuro-oncologist at the Seattle Children’s Hospital Cancer and Blood Disorders Center who led the trial, told NBC News.
Cancer Today magazine is free to cancer patients, survivors and caregivers who live in the U.S. Subscribe here to receive four issues per year.