Every week, the editors of Cancer Today magazine bring you the top news for cancer patients from around the internet. Stay up to date with the latest in cancer research and care by subscribing to our e-newsletter.
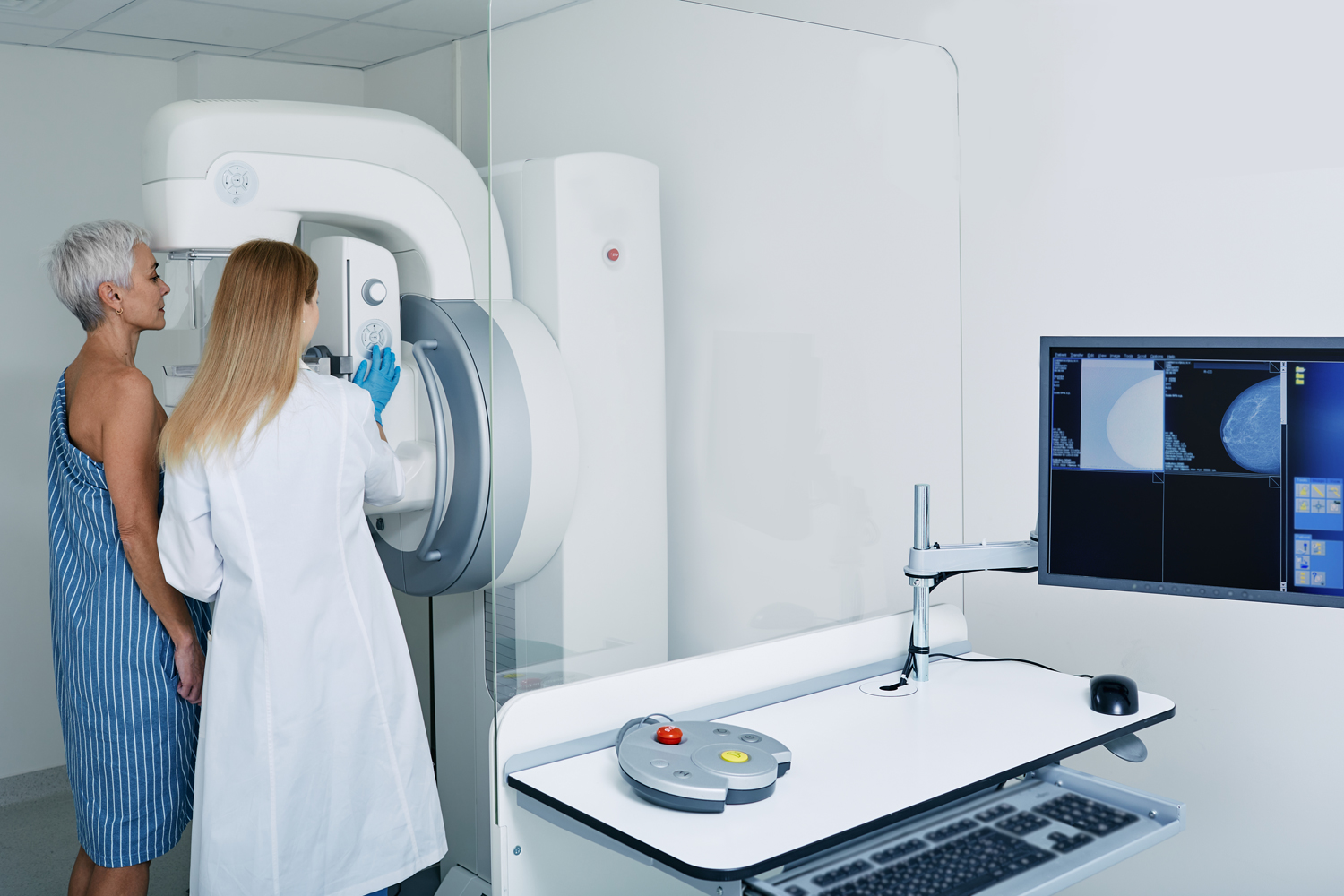
Experts Still Unsure How to Address Dense Breast Tissue
About half of women over 40 who receive regular mammograms have dense breast tissue, meaning their breasts have more fibrous and glandular tissue than fatty tissue. In a mammogram, this dense tissue and cancer tissue have a similar appearance, making tumors difficult to detect. Starting in September 2024, all screening facilities will be required to notify women if they have dense breast tissue. What they should do from there is unclear. Despite the prevalence of dense breast tissue, no major medical society offers clear guidance on how to handle screening for these women and insurance often doesn’t cover additional testing, STAT reported. Nevertheless, most of the breast-imaging experts who spoke with STAT support follow-up screening. One option is a breast ultrasound, which can spot up to 50% more cancers in these women compared with mammograms but also has a 90% false positive rate. Meanwhile, MRIs can find up to 200% more cancers compared with mammograms, but they are costly and time consuming. Some experts don’t recommend any follow-up screening for women with dense breast tissue who are at average risk for breast cancer. “We just don’t have enough evidence to support that our health care dollars should be spent this way because we’re spending them on unproven tests that are likely to have more harm than benefit,” Nancy Lynn Keating, a medical oncologist at Brigham and Women’s Hospital in Boston, told STAT.
FDA Requires Warning on CAR T-cell Therapies
The Food and Drug Administration (FDA) has ordered the makers of chimeric antigen receptor (CAR) T-cell therapies to update product labels to include a warning that the treatment itself may cause cancer. In CAR T-cell therapy, a patient’s T cells are removed from their blood, genetically engineered to attack cancer cells and then reintroduced into the body. Since 2017, the FDA has approved six CAR T-cell therapies that have been used by more than 27,000 people with blood cancers. The FDA sent letters to the manufacturers of these six drugs, informing them that they need to add a boxed warning to note the therapy carries a risk of subsequent cancers, NBC News reported. The impacted drugs are Abecma (idecabtagene vicleucel), Breyanzi (lisocabtagene maraleucel), Carvykti (ciltacabtagene autoleucel), Kymriah (tisagenlecleucel), Tecartus (brexucabtagene autoleucel) and Yescarta (axicabtagene ciloleucel). The therapies will stay on the market, and drugmakers have 30 days to submit proposed label changes to the FDA. The agency’s request comes after it announced in November 2023 that it had received 19 reports of people who had developed a new cancer after receiving CAR T-cell therapy. Despite these reports and the newly required warning, FDA spokesperson Carly Kempler told NBC News, “The overall benefits of these products continue to outweigh their potential risks.” Many experts echoed this sentiment, saying they do not expect the new warnings to affect patients’ use of these treatments.
Liquid Biopsy Has Mixed Results Predicting Colorectal Cancer Outcomes
Many questions remain about liquid biopsy’s role in cancer care—a point made evident by research presented at the 2024 American Society of Clinical Oncology Gastrointestinal Cancers Symposium, held Jan. 18 to 20 in San Francisco. While one study showed the testing helped predict disease-free survival, another found liquid biopsy ineffective in determining the best course of treatment, OBR Oncology reported. In the first study, 2,998 people with stage II to IV colorectal cancer who had just undergone surgery had a liquid biopsy to determine if they had circulating tumor DNA (ctDNA) in their blood. Two years after surgery, disease-free survival rates were 85.9% for participants who had no ctDNA and 28.9% for patients who had ctDNA. In the second study, 635 people who had undergone surgery for stage IIA colon cancer were randomly assigned to either active surveillance, which is the standard of care, or ctDNA-directed therapy. In the latter arm, participants whose liquid biopsy showed ctDNA received chemotherapy and those without ctDNA went with active surveillance. Of the 16 people with detectable ctDNA, seven were assigned to the active surveillance arm and nine received chemotherapy. After six months, 43% of the active surveillance group had no ctDNA, while 11% of the chemotherapy cohort had no ctDNA—disproving the hypothesis that those with ctDNA would see greater benefit from chemotherapy. Based on these results, researchers closed the trial. “With regards to ctDNA, we certainly have many answers, but I’ll make the argument that we have many more questions that are still left to answer,” Aparna Parikh, a gastrointestinal oncologist at Massachusetts General Hospital in Boston who was not involved in the research, said during a discussion of the two studies.
Cancer Today magazine is free to cancer patients, survivors and caregivers who live in the U.S. Subscribe here to receive four issues per year.