Every week, the editors of Cancer Today magazine bring you the top news for cancer patients from around the internet. Stay up to date with the latest in cancer research and care by subscribing to our e-newsletter.
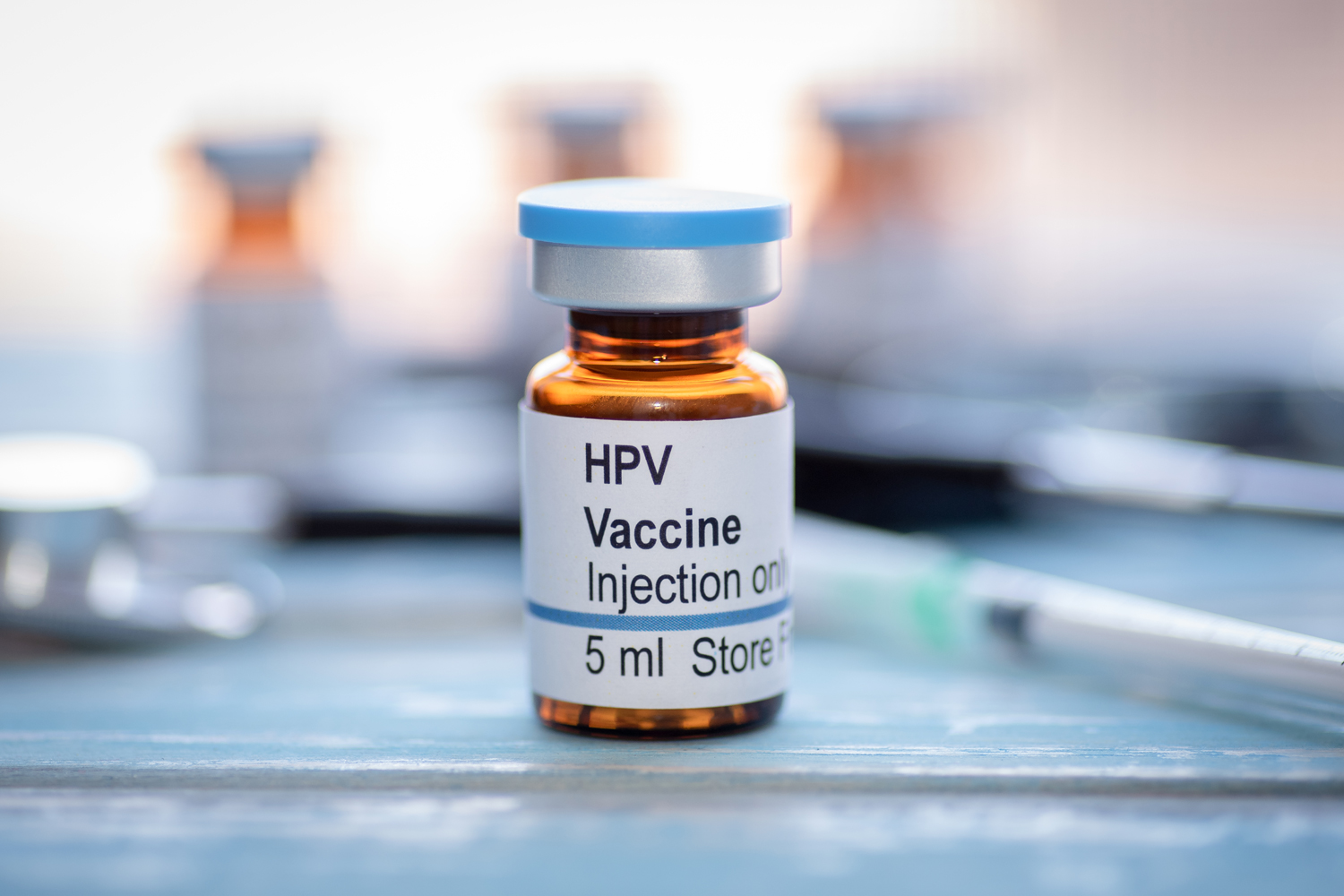
Cervical Cancer Mortality Drops in Young Women
Between 2013 and 2021, cervical cancer mortality decreased by 62% among women younger than 25, in large part due to the increased prevalence of human papillomavirus (HPV) vaccination, according to a study published in JAMA. Researchers analyzed national health data from 1992 to 2021, finding 398 women under 25 died from cervical cancer in the U.S. during that period. From 1992-1994 to 2013-2015, cervical cancer death rates dropped by 3.7% per year. That decline accelerated to 15.2% per year between 2013-2015 and 2019-2021, leading to a cumulative 62% decrease during that timeframe. Women younger than 25 during this period were among the first to widely receive the HPV vaccine, which was approved in 2006 for use in girls and women 9 to 26. The vaccine protects against certain HPV strains that cause most cervical cancers. “While previous research suggest that HPV vaccination has resulted in a decline in cervical cancer incidence, our study is the first to suggest that we have now started to see the impact of HPV vaccination on cervical cancer mortality,” Ashish A. Deshmukh, a study author and a cancer epidemiology researcher at Hollings Cancer Center in Charleston, South Carolina, told Healio. “This is an important public health milestone that signifies the importance of HPV vaccination.”
Tumor Treating Fields Improve Survival in Pancreatic Cancer
Combining tumor treating fields (TTFields) with chemotherapy improved overall survival in people with pancreatic cancer compared with chemotherapy alone, according to results of a phase III clinical trial. This technology uses a portable device attached to the skin near the tumor that emits electrical pulses to destroy cancer cells. The Food and Drug Administration approved the device in October for use in lung cancer, and researchers now are looking at how it could improve pancreatic cancer treatment. In the trial, 571 people with locally advanced pancreatic cancer that could not be surgically removed were randomly assigned to receive either TTFields and chemotherapy or chemotherapy alone, OncLive reported. Median overall survival was 16.2 months among those who used TTFields versus 14.16 months in the other group. Those who used TTFields also had 13% and 33% better one- and two-year overall survival, respectively, than those receiving chemotherapy alone. “These data for Tumor Treating Fields are very promising, especially in this difficult to treat patient population,” Vincent Picozzi, a medical oncologist at Virginia Mason Health Center in Seattle and an investigator of the clinical trial, said in a press release. Novocure, the manufacturer behind TTFields devices, announced the trial results, which have not yet been published in a peer-reviewed journal. Another clinical trial is exploring if this treatment can improve survival for people with metastatic pancreatic cancer.
FDA Approves First Drug for Hard-to-treat Pancreatic, Lung Cancers
The Food and Drug Administration (FDA) granted accelerated approval for the bispecific antibody Bizengri (zenocutuzumab) on Dec. 4. The drug is intended for use in pancreatic or non-small cell lung cancer (NSCLC) that has an NRG1 gene fusion, has progressed during a prior treatment and either has spread to distant parts of the body or cannot be surgically removed. Bizengri is the first drug approved in these cancers to treat an NRG1 gene fusion, which occurs in less than 1% of solid tumors, MedPage Today reported. The accelerated approval is based on results from a phase I/II clinical trial in which 64 people with NSCLC and 30 people with pancreatic cancer, all of whom had advanced or metastatic disease with an NRG1 gene fusion, received Bizengri. Among participants with NSCLC, 33% responded to the drug, with response continuing for a median of 7.4 months. Meanwhile, 40% of people with pancreatic cancer experienced a response, with duration ranging from 3.7 to 16.6 months. The most common side effects included diarrhea, fatigue, musculoskeletal pain and nausea. “The FDA approval of Bizengri marks an important milestone for patients with pancreatic adenocarcinoma or NSCLC that is advanced unresectable or metastatic and harbors the NRG1 gene fusion,” Alison Schram, a trial investigator and a medical oncologist at Memorial Sloan Kettering Cancer Center in New York City, said in a press release. “I have seen firsthand how treatment with Bizengri can deliver clinically meaningful outcomes for patients.”
Cancer Today magazine is free to cancer patients, survivors and caregivers who live in the U.S. Subscribe here to receive four issues per year.