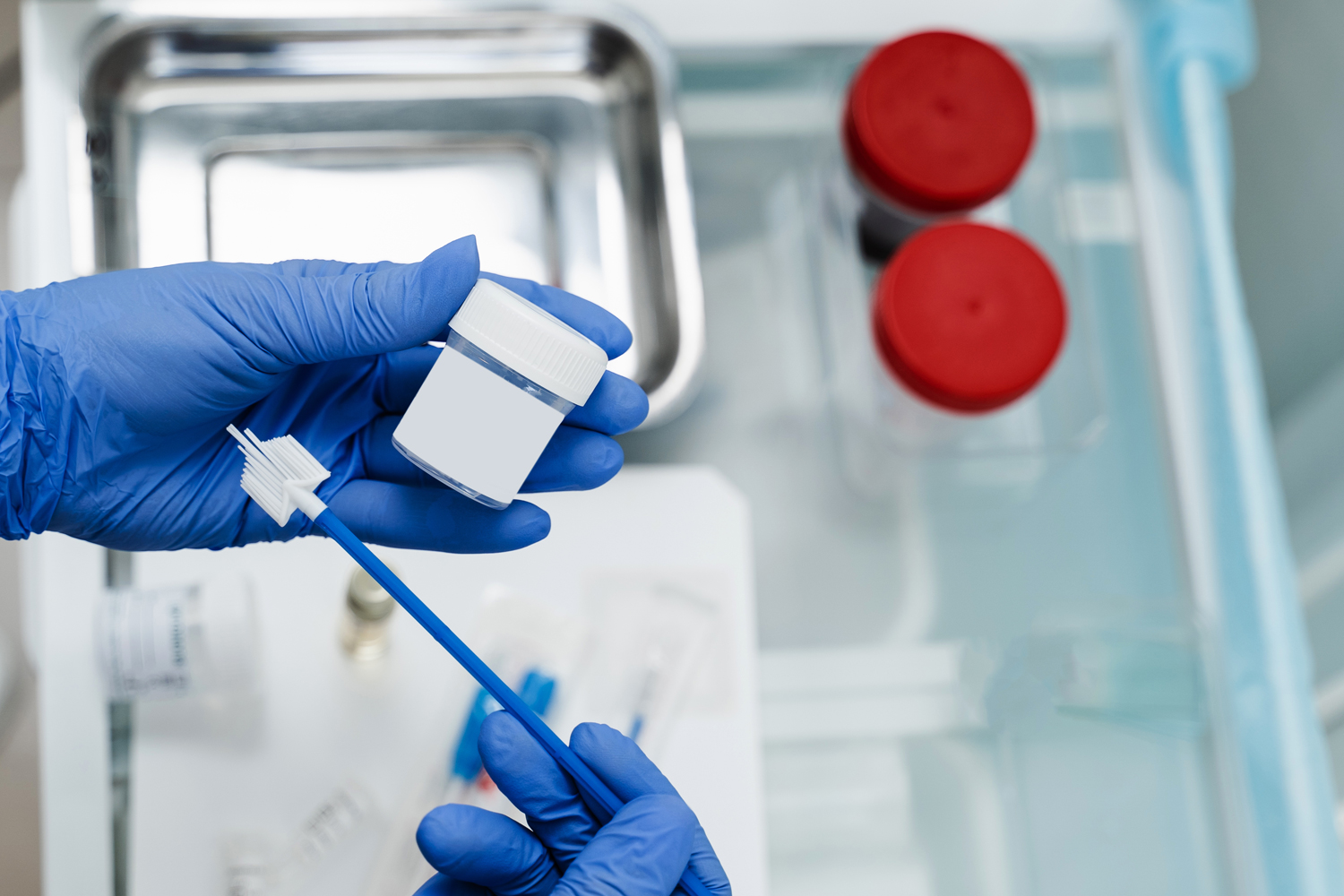
THE FOOD AND DRUG ADMINISTRATION (FDA) expanded its approval for two human papillomavirus (HPV) tests allowing patients to self-collect samples. The May 14 announcement could improve the rate of routine screenings and lower cervical cancer risk in the United States.
According to the Centers for Disease Control and Prevention (CDC), more than 42 million Americans are infected with strains of HPV that can cause disease. While most HPV infections disappear on their own within two years, some can last longer, possibly leading to cancer in the cervix, vagina, vulva, penis, anus and back of the throat. The CDC reports that more than nine out of every 10 cases of cervical cancer are caused by HPV.
Two screening methods to check for cervical cancer are HPV and cytology tests, also known as Pap tests or Pap smears, according to the National Cancer Institute.
HPV screening could previously be given at doctor’s offices, community health clinics, and local health departments. However, many primary care providers didn’t offer testing.
“Whether primary care providers perform human papillomavirus (HPV) screening can depend on various factors,” says Heather Thompson Mackey, the senior director of cancer prevention and early detection at Prevent Cancer Foundation in Alexandria, Virginia. “For example, the practice may not have the resources to conduct these screenings. Sometimes, conflicting professional organization guidelines on testing leads to confusion, or there is a lack of awareness that HPV testing is now recommended.”
Mackey says these issues can create a barrier for patients who have to seek HPV screening from a specialist or other health care provider.
“[Potential barriers can] involve scheduling with a different health care practice and potentially traveling further for care as well as taking additional time off from work or caregiving responsibilities for another appointment,” Mackey says.
HPV Self-collection Test Approval
The FDA approval expansions for two HPV tests allow patients to collect samples for the tests themselves, though still in a health care setting. Anuj Suri, a gynecologic oncologist at Houston Methodist Hospital in Houston, says this is an important development in cervical cancer screening. “These [samples] can be collected privately and sent to the appropriate lab for processing.”
The 1987-2021 National Health Interview Survey conducted by the CDC and the National Center for Health Statistics reported that in 2021, an estimated 72.4% of females between the ages of 21 and 65 were up to date with cervical cancer screening.
According to Mackey, there are many reasons why someone may not be getting their recommended HPV screening, including lack of awareness, anxiety about the exam or potential results, difficulty getting transportation to a provider, and concerns about insurance coverage. The FDA’s decision could ease some of these burdens.
“[HPV self-collection tests] may benefit patients who have anxiety or discomfort seeing a physician or have a history of trauma or particular religious or cultural beliefs,” Suri says. “These tests have the opportunity to decrease the barriers to testing and help take care of certain patient populations.”
One study found that self-collection tests can encourage more patient involvement and ownership of their health and improve the patient-provider relationship with shared decision-making about their care.
And while this approval limits self-collection to health care settings, Mackey says advocates are working to expand approval to at-home collection as well.
“HPV screening is paramount to preventing cancer, so the more options we can give people, the better,” Mackey says.
How Self-collection May Affect Cancer Prevention and Detection
Self-collection tests can help reduce the risk of cancer with early detection of HPV infections that have the potential to cause cancer.
“Through HPV testing, we can identify those at greater risk and make sure they are getting the care they need, including finding cancers early—leading to better outcomes,” Mackey says.
However, Suri says health care providers must deliver proper health education to patients for these self-collection methods to be effective.
“The efficacy of the test and the proper interpretation of the results will be a very important factor in the success of these kits,” Suri says. “Patients need to understand that with positive results, they will likely need to see a physician for further evaluation and examination. [Their physician] may require further testing, such as cervical colposcopy and biopsies.”
Mackey says that self-collection is supplemental to HPV screening and does not replace regular screening methods.
“If you have a cervix and are of average risk for cervical cancer, you should follow the current guidelines for cervical cancer screening,” Mackey says. “Begin screening at age 21 with a Pap test every three years. From ages 30 to 65, you can continue to have a Pap test alone every three years, have an HPV test alone every five years, or have a combination of HPV testing with a Pap test (called co-testing) every five years.”
Cancer Today magazine is free to cancer patients, survivors and caregivers who live in the U.S. Subscribe here to receive four issues per year.