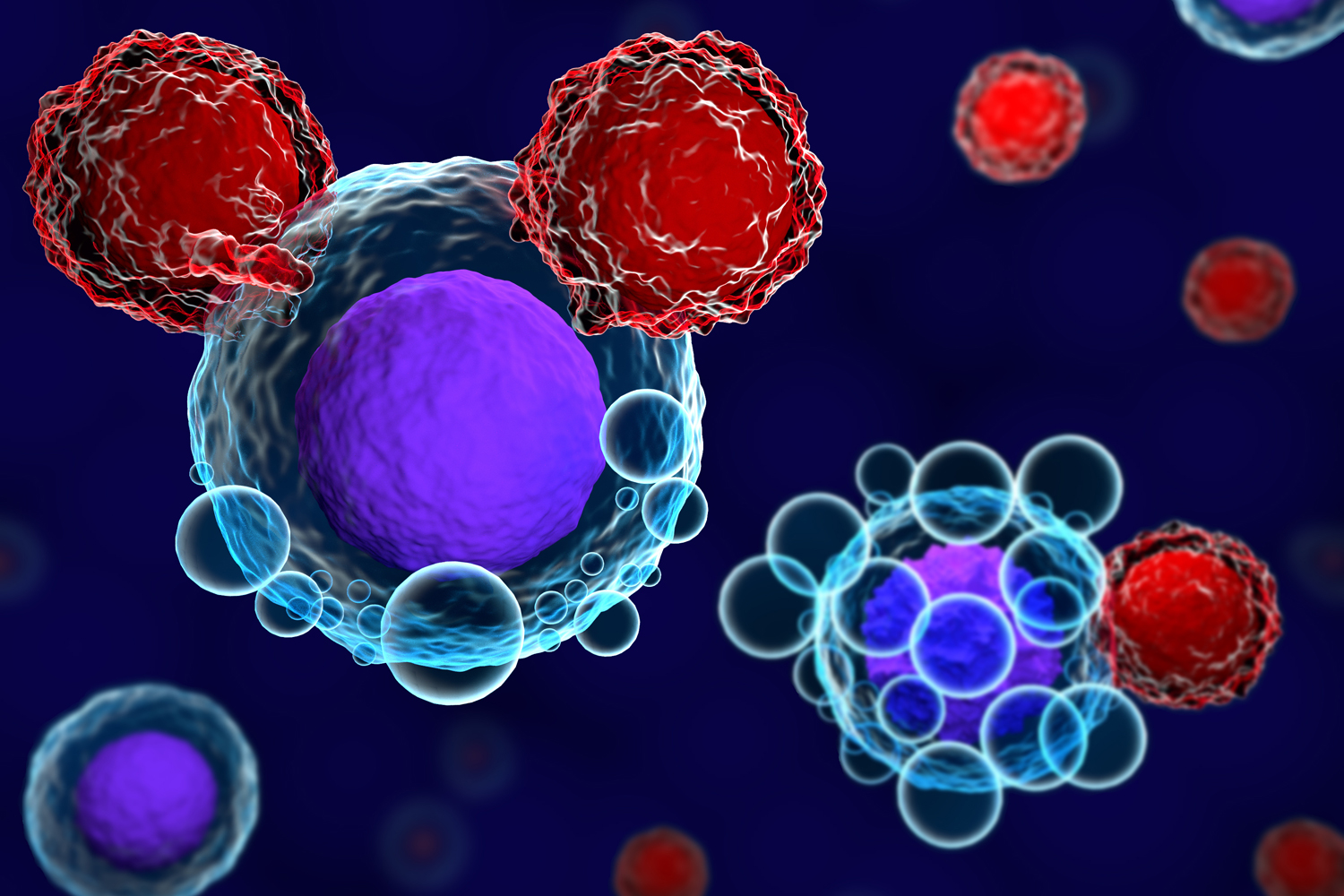
FDA Approves First CAR T-cell Therapy for CLL
The Food and Drug Administration (FDA) has granted accelerated approval for a chimeric antigen receptor (CAR) T-cell therapy for relapsed or refractory chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL), marking the first CAR T-cell therapy approval for these diseases. In CAR T-cell therapy, a patient’s immune cells are extracted from their blood, genetically modified in the lab to target cancer cells and then reintroduced into the bloodstream. On March 14, the FDA approved Breyanzi (lisocabtagene maraleucel) for treatment of adults with CLL or SLL who have tried at least two other therapies, MedPage Today reported. The approval was based on results of a phase I/II clinical trial, in which 65 people with CLL or SLL received Breyanzi. Among these participants, 45% of cancers responded to treatment, with 20% having a complete response. All those who went into remission had no signs of cancer one year later. More than 18,000 people in the U.S. are diagnosed with CLL annually. While BCL-2 and BTK inhibitors have proven to be effective therapies for CLL, Breyanzi is the first approved treatment for people whose cancers do not respond to either of those drugs. This approval “is a remarkable breakthrough, shifting the treatment paradigm from continuous therapy with sequential regimens to overcome drug resistance, to a one-time personalized T-cell based approach that has the potential to offer patients complete and lasting remission,” Tanya Siddiqi, the trial’s lead investigator and a hematologist at City of Hope National Medical Center in Duarte, California, said in a press release.
EPA Bans Final Type of Asbestos Used in U.S.
The Environmental Protection Agency (EPA) has announced it will ban the last form of asbestos currently used in the U.S., bring the country in line with more than 50 other nations that have banned the carcinogenic material. With its March 18 announcement, the EPA proposed a rule that would immediately ban imports of chrysotile asbestos and phase in prohibitions on its use over the next five years, the Associated Press reported. The EPA outlawed asbestos in 1989, but a 1991 court ruling found the agency lacked the authority to do so. Then 2016’s Frank Lautenberg Chemical Safety Act granted the EPA expanded oversight of chemicals that threaten human health, allowing most types of asbestos to be banned. However, chrysotile asbestos is still used in some brake pads and the making of chlorine bleach and other chlor-alkali products, although most uses for this form of asbestos have been discontinued. Asbestos exposure can cause lung cancer and mesothelioma, a cancer that forms in the lining of the abdomen or chest, and has been linked to more than 40,000 deaths annually in the U.S. “With today’s ban, EPA is finally slamming the door on a chemical so dangerous that it has been banned in over 50 countries,” EPA Administrator Michael Regan told the Associated Press. “This historic ban is more than 30 years in the making.”
Study Finds Bacteria Subspecies in Half of Colorectal Cancer Tumors
A subspecies of bacteria that can negate the efforts of cancer therapy was found in half of colorectal cancer tumors, according to a study published online March 20 in Nature. Researchers analyzed the bacterial composition of 116 colorectal tumors and stool samples from 1,246 people, about half of whom had colorectal cancer. They found 50% of the tumors contained a subtype of Fusobacterium nucleatum, a bacteria commonly found in the mouth that can cause plaque and gum disease, NBC News reported. Also, stool samples from people with cancer had higher amounts of this bacteria compared with those of individuals without the disease. When transplanted into mice, this specific subspecies caused precancerous polyps to form, indicating it may play a role in colorectal cancer development. Additionally, this subtype of bacteria “acts like a cloak” and helps shield colorectal tumors from immune cells, making the cancer resistant to treatment, Susan Bullman, the study’s co-lead author and a researcher at the Fred Hutchinson Cancer Center in Seattle, told NBC News. “Patients who have high levels of this bacteria in their colorectal tumors have a far worse prognosis,” she said. “They don’t respond as well to chemotherapy, and they have an increased risk of recurrence.” Researchers said the findings could lead to treatments or preventive antibiotics that target this specific form of bacteria, while initial colorectal cancer screening could eventually include a mouth swab.
Cancer Today magazine is free to cancer patients, survivors and caregivers who live in the U.S. Subscribe here to receive four issues per year.