Every week, the editors of Cancer Today magazine bring you the top news for cancer patients from around the internet. Stay up to date with the latest in cancer research and care by subscribing to our e-newsletter.
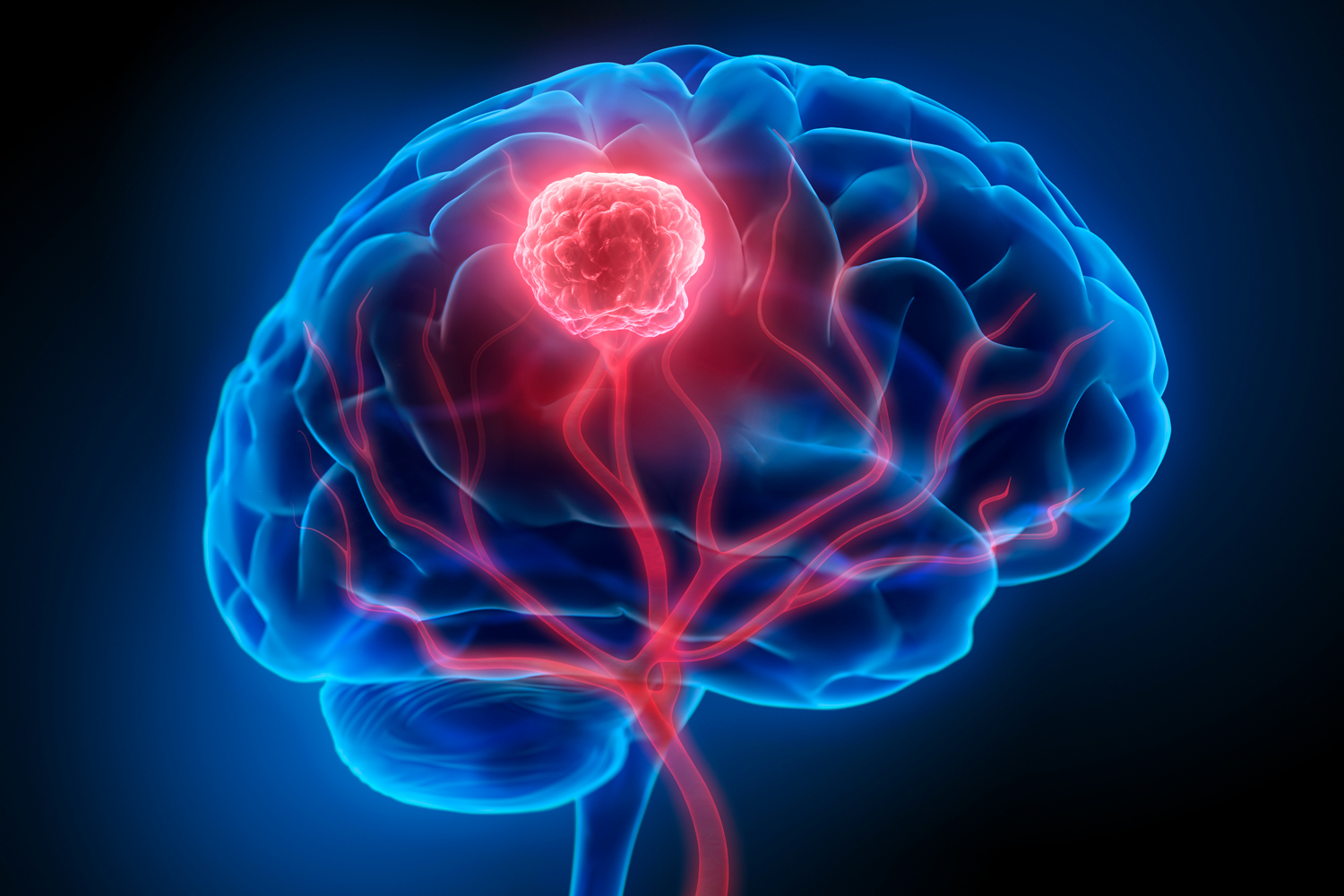
CAR T-cell Therapy Elicits Temporary Responses in People With Glioblastoma
Researchers observed tumors shrink within days of injecting modified versions of CAR T-cell therapy into people with glioblastoma in two small, phase I studies published on March 13, NBC News reported. Glioblastoma is an aggressive brain cancer with a five-year survival rate of about 10%. CAR T-cell therapy, which takes immune cells from the patient that are then modified and reintroduced to attack cancer cells, is used in several blood cancers and can produce lasting results in many patients, but it has yet to be shown effective in treatment for solid tumors. The two trials published this week were unrelated and very small, together treating just nine patients. Though the results were mostly temporary, they raised hopes that CAR T-cell therapy could be a potential pathway for treatment in a disease that has not had a new medication in over a decade, according to the NBC News story. One trial, published online in the New England Journal of Medicine, treated three people with a CAR T-cell therapy targeting two variants of a protein that is common in glioblastoma tumors. The tumors shrank within days in all three patients, but the results were temporary—the cancer returned within six months for two of the patients. An unrelated study published in Nature Medicine tested six people with a CAR T-cell therapy after first shrinking the cancer with radiation. Researchers found a rapid growth in the number of active CAR T cells in the spinal fluid that lasted for weeks and also observed a reduction in tumor size for all patients within days of treatment, though the authors noted the tumor effects did not meet their criteria for objective response. Marcela Maus, director of the Cellular Immunotherapy Program at the Massachusetts General Cancer Center in Boston and lead author on the New England Journal of Medicine study, told NBC News that CAR T-cell therapy may be more effective in glioblastoma if the cancer is first weakened with radiation, as done in the Nature Medicine trial. “We’re learning from each other. I think that’s really a tremendous model, and what we are all seeing is that this sort of therapy has legs for brain tumor patients,” Maus said. “It may not be the final version yet, but we’re onto something.”
Injection Could Make Immunotherapy More Accessible
An injectable form of the immunotherapy Opdivo (nivolumab) appears to have similar effectiveness as the IV formulation, based on early results from a clinical trial published Jan. 29 in the Journal of Clinical Oncology. Opdivo is currently given by IV infusion, which can last more than 30 minutes and require getting to an infusion center. Subcutaneous injection, a shot given through the skin, can be administered in just five minutes in a doctor’s office. Patients are “already battling fitting [cancer treatment] into normal life and balancing work and life,” said Mark Ball, a kidney cancer expert in the National Cancer Institute’s Center for Cancer Research who was not involved in the trial. He told Cancer Currents, a blog by the National Cancer Institute, that having an injectable form of a drug to treat many cancers has the potential to improve patients’ quality of life. The study, which looked at 500 patients with advanced or metastatic kidney cancer, found the injection was similar in treatment effectiveness and side effects to the IV version of the medication. The cancer shrank or disappeared in 24% of patients given the injection and 18% of those who got IV infusion. Patients given the injection also went seven months without cancer getting worse compared with six months in those who got IV treatment. Opdivo is approved to treat several cancers besides kidney cancer, including melanoma, Hodgkin lymphoma and colorectal cancer, and more research may be needed to confirm that this effect is seen across cancer types.
Metabolic Syndrome Linked to Higher Cancer Risk
People with metabolic syndrome—a condition that can include high blood pressure, high levels of triglycerides, high blood sugar, low levels of high-density lipoproteins (HDL) and extra fat around the abdomen—may have a higher risk of developing cancer, according to a study published online March 11 in Cancer. Included in the study were more than 40,000 people in China who had some or all of the five markers of metabolic syndrome. Those with metabolic syndrome—defined as having at least three of those markers, including extra fat around the abdomen—had a 30% higher risk of developing any type of cancer than those who did not. Sheetal Hardikar, a researcher at the Huntsman Cancer Institute who was not involved with this study, told NPR that it will be important for future research to look at the role of each of the metabolic factors beyond weight. Her lab has found people with a smaller waist but other metabolic issues also had an elevated risk for cancer that appeared even higher than those with large waists and metabolic syndrome. “We do know that there are at least 13 cancers that are causally-related to obesity. But there are other things like [high blood sugar], hypertension that may also be contributing,” Hardikar said.
Cancer Today magazine is free to cancer patients, survivors and caregivers who live in the U.S. Subscribe here to receive four issues per year.