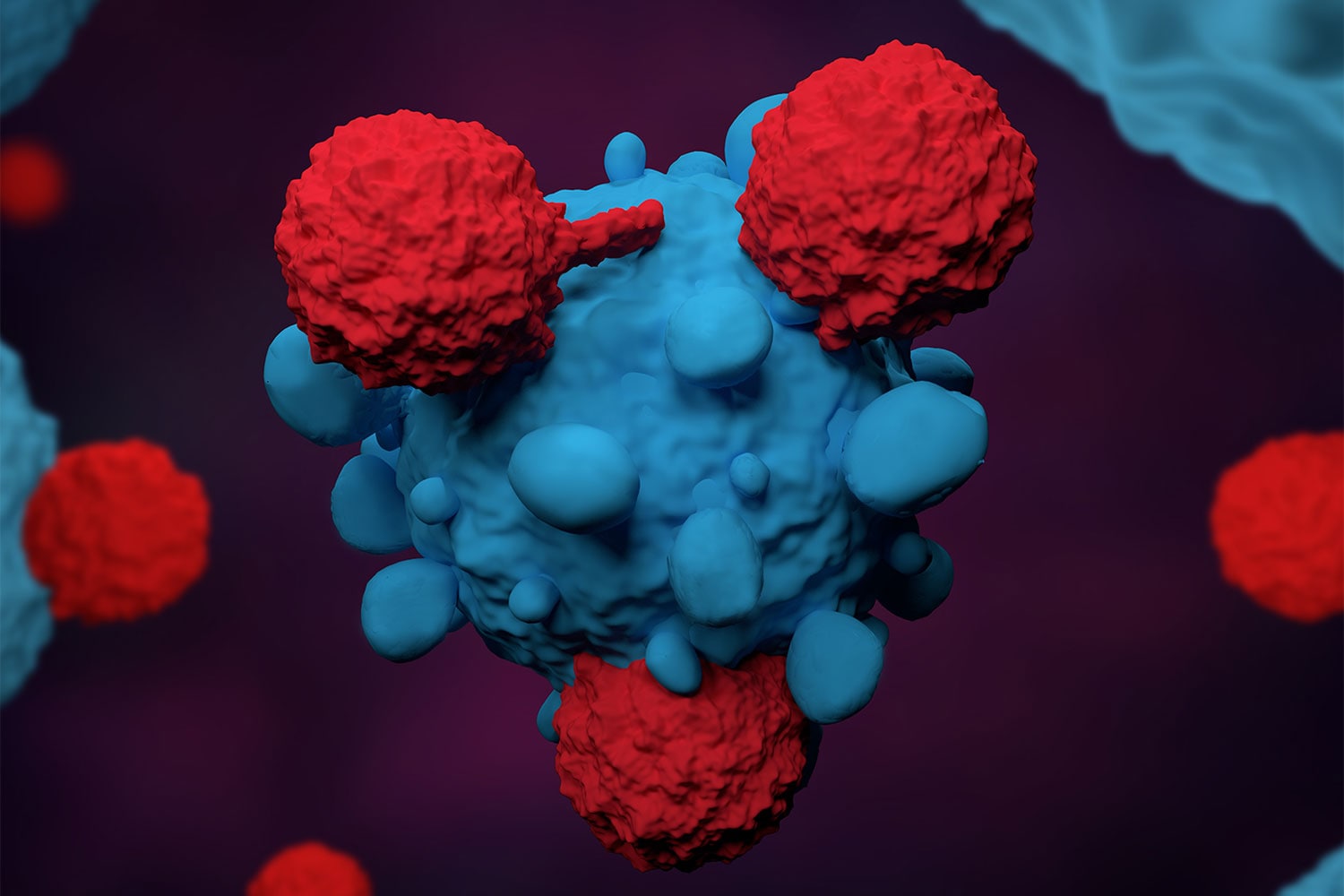
Opdivo-Yervoy Combination Shows Lasting Effectiveness in Treating Renal Cell Carcinoma
Data presented at the European Society for Medical Oncology virtual meeting Sept. 16 indicate that the combination of two immunotherapy drugs, Opdivo (nivolumab) and Yervoy (ipilimumab), continues to be effective for patients with advanced or metastatic renal cell carcinoma (RCC), the most common type of kidney cancer. Results from the phase III CheckMate-214 trial show a five-year overall survival rate of 48% for patients with previously untreated advanced or metastatic RCC. The median overall survival for patients with intermediate or poor risk was 47 months, compared with 26.6 months for patients taking Sutent (sunitinib), an existing treatment. “The five-year data from the CheckMate-214 trial demonstrate that nearly half of patients lived longer after five years in the phase III trial for advanced renal cell carcinoma,” said medical oncologist and study investigator Robert J. Motzer of Memorial Sloan Kettering Cancer Center in New York City in a press release. Other studies have shown that the Opdivo-Yervoy combination has also been effective in treating non-small cell lung cancer, metastatic melanoma, malignant pleural mesothelioma and esophageal squamous cell carcinoma.
Patients Treated with Immune Checkpoint Inhibitors Report on Their Quality of Life
An analysis published Sept. 11 in the Journal of the National Cancer Institute concludes that cancer patients being treated with immune checkpoint inhibitors, a type of immunotherapy, report a higher quality of life compared with patients treated with other types of therapy. Researchers reviewed 26 published studies that analyzed patients’ self-reported changes in quality of life, physical functioning and symptoms when being treated with immune checkpoint inhibitors. They found that quality of life did not change significantly for most patients undergoing treatment with these therapies. An exception was reported by patients being treated with Yervoy (ipilimumab), especially melanoma patients, who reported worse quality of life. Overall, patients receiving checkpoint inhibitors reported better quality of life than patients being treated with other therapies. “A growing number of patients are receiving immune checkpoint inhibitors and need evidence-based information regarding what to expect on treatment,” said lead author Brian D. Gonzalez, a researcher at Moffitt Cancer Center in Tampa, Florida, in a press release. “This study is among the first to aggregate data about patient-reported outcomes of immunotherapy.”
FDA Approves Oral Treatment for Some Lung Cancer Patients
The Food and Drug Administration (FDA) granted accelerated approval Sept. 15 to Exkivity (mobocertinib), the first oral treatment for adult patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) that features epidermal growth factor receptor (EGFR) exon 20 insertion mutations. The FDA also approved the Oncomine Dx Target Test, a companion diagnostic that identifies patients with these mutations. In a phase I/II study presented at the 2021 American Society of Clinical Oncology meeting in May, Patients in the trial had previously been treated with platinum-based chemotherapy. NSCLC is the most common form of lung cancer, accounting for about 85% of cases, although patients with EGFR exon 20 insertions make up only 1% to 2% of NSCLC patients.
Cancer Today magazine is free to cancer patients, survivors and caregivers who live in the U.S. Subscribe here to receive four issues per year.