THE SUMMER BEFORE starting sixth grade, in 2006, Taylor Hendrix felt twinges in her right arm and shoulder after swim practice. The 11-year-old from Florence, Alabama, chalked it up to a pulled muscle, took Tylenol to ease the discomfort and finished the swim season.
But two weeks into the school year, Hendrix was on the playground when she felt her right arm go “sort of numb,” similar to but more intense than the pins-and-needles sensation one feels when blood returns to an arm or leg that has gone to sleep.
Hendrix’s mother took her to a bone and joint clinic for an X-ray. When the doctor came in to give them the results, Hendrix could tell from the look on his face that something was going on—something he wasn’t prepared to say to an 11-year-old girl and her mother. The doctors referred Hendrix to Children’s of Alabama, a pediatric hospital in Birmingham, for a battery of tests, including a CT scan, an MRI and bloodwork. The following week, her mother received a call that would change their lives. Hendrix had osteosarcoma, a rare form of cancer that tends to occur in the long bones of the arms or legs in young adults, teens and preteens like Hendrix.
Three days after learning of her diagnosis, Hendrix started a three-month regimen of chemotherapy that was followed by surgery to remove the tumor and part of her right humerus, the long bone that extends from the shoulder to the elbow. Doctors reconstructed her arm with bone from a donor and a joint made of titanium. The surgery was followed by another six months of chemotherapy.
For the next 18 months, it seemed as though the treatment had worked. Doctors told Hendrix that she showed no evidence of disease, but they recommended another surgery to graft bone from her leg into her arm because the donor bone wasn’t fusing correctly. The mother and daughter didn’t like the sound of that, so they sought a second opinion at the University of Texas MD Anderson Cancer Center in Houston. A CT scan taken there turned up unexpected bad news: two spots on Hendrix’s right lung.
The five-year survival rate for children and teens diagnosed with osteosarcoma that hasn’t spread is between 60% and 80%. In those diagnosed after osteosarcoma has spread to the lungs, the five-year survival rate is approximately 40%. Hendrix, who is now 24 years old, had surgery to remove the cancer, followed by a series of chemotherapies and other treatments with names that are now a blur to her. Each time she was treated, the cancer seemed to respond, only to relapse about a month later. Then, new scans showed the cancer was in her lung’s lining. Getting it out would mean undergoing surgery to remove both lobes of her right lung.
Before her surgery, her oncologist at MD Anderson sent her to see Nabil Ahmed, a pediatric oncologist at Baylor College of Medicine, also in Houston. If her cancer was HER2-positive, meaning that its cells expressed an abundance of the HER2 protein on their surfaces, Hendrix would be eligible for a phase I clinical trial that was testing the safety of an experimental treatment.
A test showed the cancer was HER2-positive. Ahmed explained to Hendrix in words and pictures that her T cells, white blood cells that protect the body from pathogens and other health threats, didn’t know how to recognize and fight off her cancer. He was going to remove some of her T cells, give them the tools they needed to fight, and infuse them into Hendrix. Approximately 14 weeks after receiving the infusion, she would undergo surgery to remove the cancer and her right lung. While the trial was primarily intended to test the safety of the modified cells, one hope was that those newly infused T cells might take up residence in Hendrix’s body and attack the cancer cells. As Ahmed explained the process, Hendrix remembers thinking, “Yes, I need that. I need a lot of that.”
Making History
The experimental treatment Ahmed used on Hendrix is called CAR-T cell therapy, short for chimeric antigen receptor T cell therapy. In CAR-T cell therapy, a patient’s T cells are collected from the blood and genetically modified to express a receptor that recognizes and binds to an antigen found on the surface of cancer cells. Once modified, the CAR-T cells are reproduced in large numbers in the lab before they are infused into the patient, either intravenously or at the tumor site, where, if all goes well, they attack the cancer.

Clinicians collect immune cells, including T cells, from a patient’s blood. The remaining components of the blood are returned to the patient, while the immune cells are sent to a laboratory.
Today, CAR-T cell therapy is still considered experimental for patients like Hendrix who have sarcomas, but the U.S. Food and Drug Administration (FDA) has approved this therapy to treat some blood-related cancers. In August 2017, the FDA for the first time approved the CAR-T cell therapy Kymriah (tisagenlecleucel) to treat certain pediatric and young adult patients with a form of acute lymphoblastic leukemia (ALL). In October 2017, another CAR-T cell therapy, Yescarta (axicabtagene ciloleucel), was approved for treating adult patients with certain types of large B-cell lymphoma. (Kymriah was later also approved for certain adult lymphoma patients.) The treatment for these blood-related cancers isn’t without risks, with some patients experiencing dangerous side effects. However, clinical trials on CAR-T cells in these cancer types have shown complete remissions in 70% to 94% of patients.
The primary difference between the approved therapies and the experimental treatment Hendrix received in 2012 is the target. Instead of focusing on HER2, Kymriah and Yescarta target CD19, an antigen found on both healthy and malignant B cells. CAR-T cells can wipe out healthy B cells, which help to fight infection, but physicians can offset these effects by administering immunoglobulins, which replace the body’s supply.
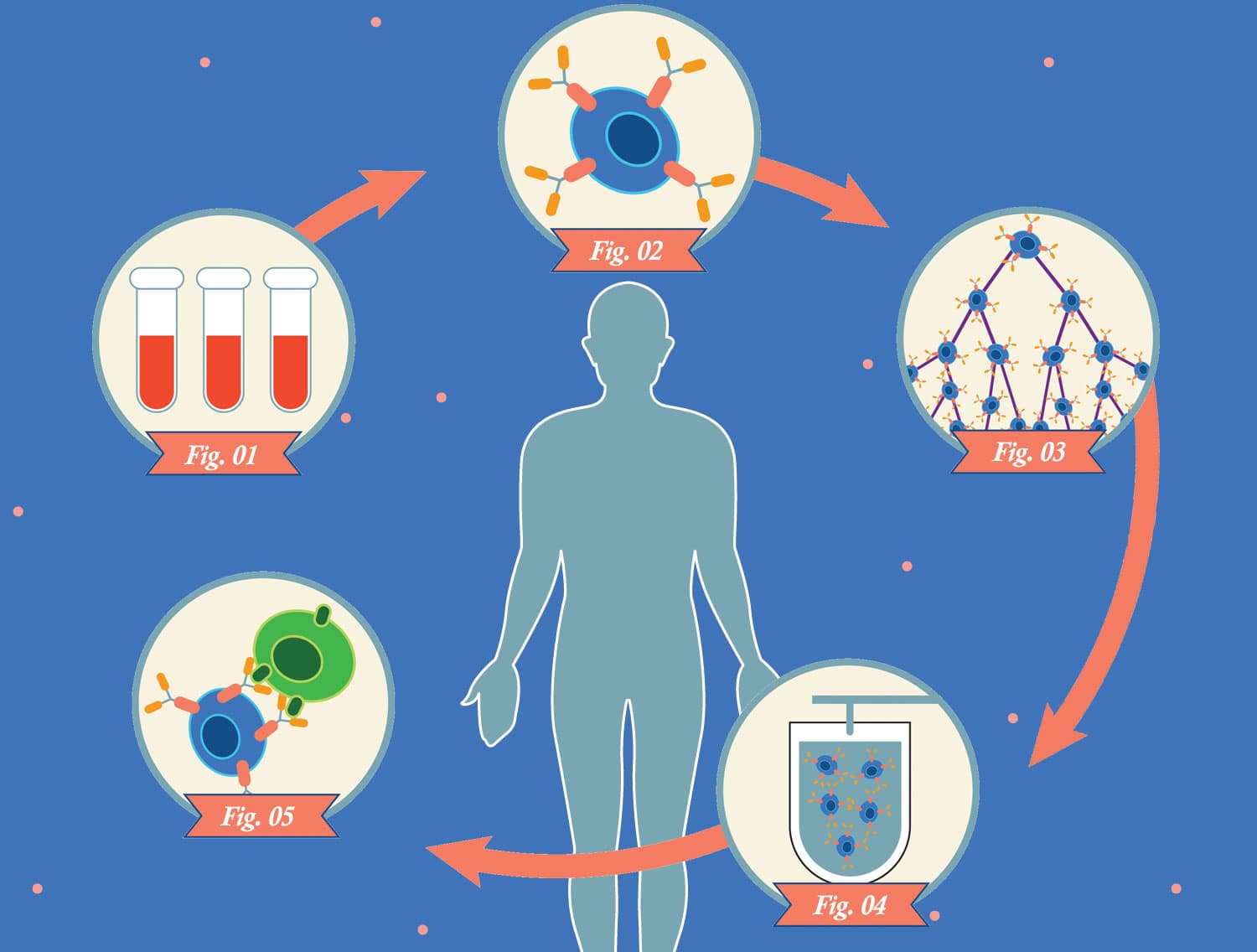
Technicians genetically modify the patient’s T cells so they express chimeric antigen receptors (CARs) on their surfaces. These receptors consist of parts of normal receptors found on T cells combined with pieces of immune proteins called antibodies.
Michael Milone, a pathologist at the Hospital of the University of Pennsylvania in Philadelphia, says one of the biggest challenges in developing CAR-T cell therapy for solid cancers is a lack of widely expressed targets that are present on cancer cells but not on essential tissues in the body. (For this article, we characterize leukemias and lymphomas as liquid cancers since they circulate in the blood or lymphatic fluid, even though lymphoma can present as a solid cancer.)
There are other challenges too. The cells within solid cancers tend to be highly variable, which makes it tough if not impossible to kill a tumor in its entirety by taking aim at any one target. In the case of leukemia or lymphoma, intravenously infused CAR-T cells can hunt down and attack cancer cells circulating in the bloodstream or lymphatic fluid. In solid cancers, modified T cells have a tougher time finding their way to the tumor. If and when they do, the environment commonly found inside solid tumors makes CAR-T cells’ long-term survival difficult.
“These are hurdles, not obstacles,” Ahmed said. “It should not stop our progress, but we need to have all of these [factors] accounted for. We need to have good research and development and go realistically into the trenches.”
An Encouraging Start
There is plenty of research and development now underway, buoyed by the success of CAR-T cell therapy in blood-related cancers and early hints of safety and efficacy in some patients with other types of cancers. At the San Antonio Breast Cancer Symposium in December 2018, Jennifer Specht, a medical oncologist at Fred Hutchinson Cancer Center in Seattle, reported data from a phase I trial on CAR-T cells that targeted a tumor receptor called ROR1 in previously treated patients with metastatic triple-negative breast cancer (TNBC) or non-small cell lung cancer.
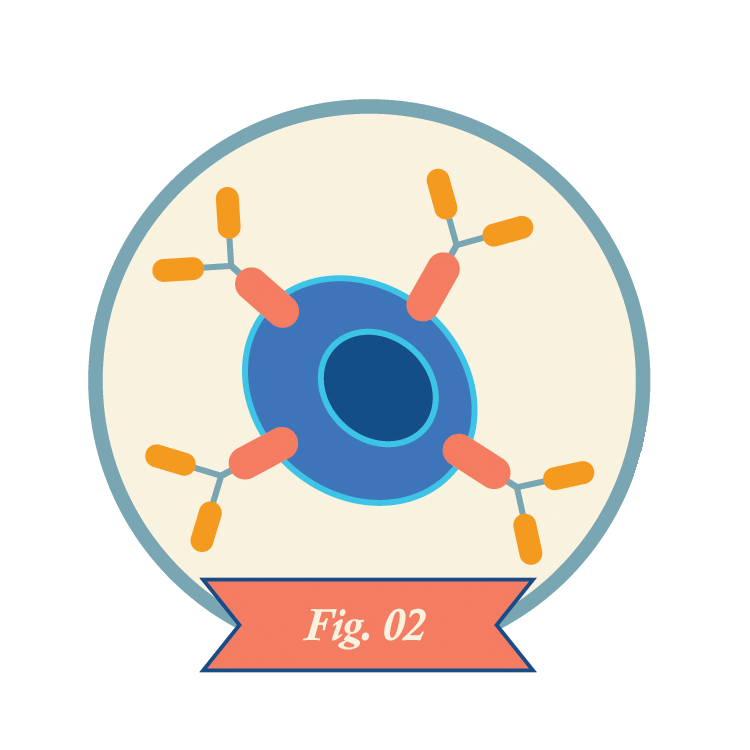
Technicians multiply the CAR-T cells in the lab, often yielding hundreds of millions of cells.
While the point of a phase I trial is to test treatment safety at various doses, early results from this trial indicated that CAR-T cell therapy targeting ROR1 has promise. At the time of Specht’s presentation, none of seven patients with TNBC who had initially enrolled in the trial experienced severe toxicity from the treatment. In addition, researchers were able to detect a small but significant increase in the number of CAR-T cells in some patients. As evidence that the cells reached the tumor, two patients in the trial had stable disease more than three months after receiving CAR-T cells.
“We are encouraged,” Specht says. “We’ve seen a couple patients with fairly durable partial responses, the shrinkage of tumors, and others with prolonged stable disease in which the tumor seemed to be held in check. It’s too early to say if this is something tremendously successful.”
The trial is ongoing with plans to increase the dose of CAR-T cells delivered. The researchers are also looking at ways to better support the CAR-T cells’ survival and expansion after they’ve been infused.
Other early clinical trials of CAR-T cell therapy are underway or soon to start for mesothelioma, glioblastoma, pancreatic cancer and prostate cancer. In general, the treatments have been well tolerated so far, but it remains an open question whether that will continue to hold true as the doses are increased in future trials.
Researchers are working to make CAR-T cell therapies safer for patients.
Despite the success of CAR-T cell therapy in certain types of leukemia and lymphoma, patients who undergo this treatment need to be carefully monitored for toxicities. In fact, as many as half of patients enrolled in early CAR-T cell trials required intensive care management.
The most common side effect of CAR-T cell therapy is cytokine release syndrome (CRS), an inflammatory response that typically occurs as the CAR-T cells multiply in the body approximately one to 21 days after infusion. Symptoms of CRS are similar to flu, including fever and shakes, and generally can be managed by the patient’s health care team. However, these symptoms can become more severe, leading to multiple organ failure and even death in rare cases. Some people receiving CAR-T cell therapies may also experience neurological symptoms, including confusion and problems with speech. Researchers are working to predict when treatment side effects become dangerous, as well as to identify the best management approaches.
Scientists are also working on engineering a kinder CAR-T cell therapy. In June 2019, researchers reported in Nature Medicine the results of a phase I clinical trial including 25 patients with B-cell lymphoma treated with a range of doses of a modified anti-CD19 CAR-T cell therapy that produces lower levels of cytokines. Six patients in a subset of 11 who received a certain dose went into remission, and none of the patients in the trial developed neurotoxicity or CRS.
Another approach is to develop CAR-T cells with more sophisticated receptors that would enable the T cells to recognize and respond to cancerous cells more precisely without going after targets in healthy tissue.
On the Cusp
Researchers at City of Hope National Medical Center in Duarte, California, are working to bring CAR-T cell therapy to patients with glioblastoma, an aggressive brain tumor. For this treatment, the cells are engineered to target IL13Rα2, which is overexpressed in glioblastoma and other brain cancers.
Christine Brown, a scientist who focuses on immune-oncology at City of Hope, says that the trials, while still focused primarily on safety, have already taught them a lot. Based on this early experience, the team is now doing research on other approaches that might be used in combination with IL13Rα2 to combat the highly heterogeneous nature of glioblastoma. The goal, says Brown, is to try to “box in” the cancer with suites of CAR-T cells tailored to attack multiple targets at once. Brown says there’s some evidence that CAR-T cell therapy that targets one receptor may also direct the immune system to launch a larger attack on other targets as well.
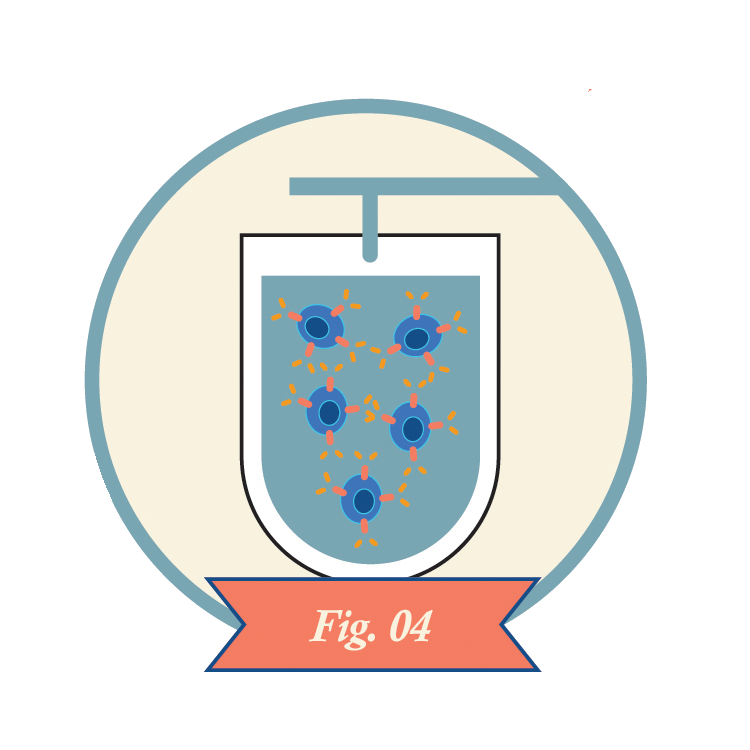
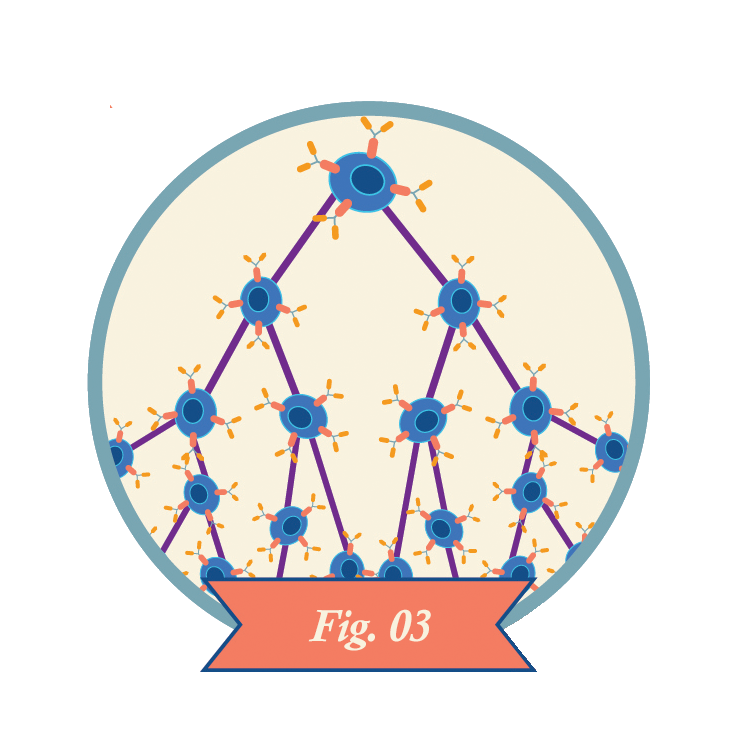
Clinicians infuse CAR-T cells into the patient.
In December 2016, Brown’s team published a case report in the New England Journal of Medicine on one of their trial participants, a 50-year-old man with recurrent, metastatic glioblastoma. After multiple infusions of CAR-T cells, the tumors in his brain and spine shrank and then disappeared, and researchers noted increases in immune activity in the cerebrospinal fluid. The clinical response continued, allowing the man to live a relatively normal life and return to work for seven and a half months. One of the reasons his complete response was especially remarkable, Brown says, is that not all of the cancers cells expressed the targeted receptor, suggesting the treatment had primed his immune system to fight cancer cells that didn’t express the target. Ultimately, however, his cancer came back in a form that resisted the treatment.
When it comes to targeting solid cancers with CAR-T cell therapy, Ahmed says, it’s clear that having two targets for treatment is better than one, and three is better than two. “There’s not a single magic bullet or Achilles-like target [in solid cancers] like CD19,” Ahmed said. “I don’t think that exists in solid tumors.” However, scientists are still trying to determine which treatment combinations will provide lasting results, including regimens adding FDA-approved checkpoint inhibitors that unleash the immune system by targeting proteins on the cancer and immune cells.
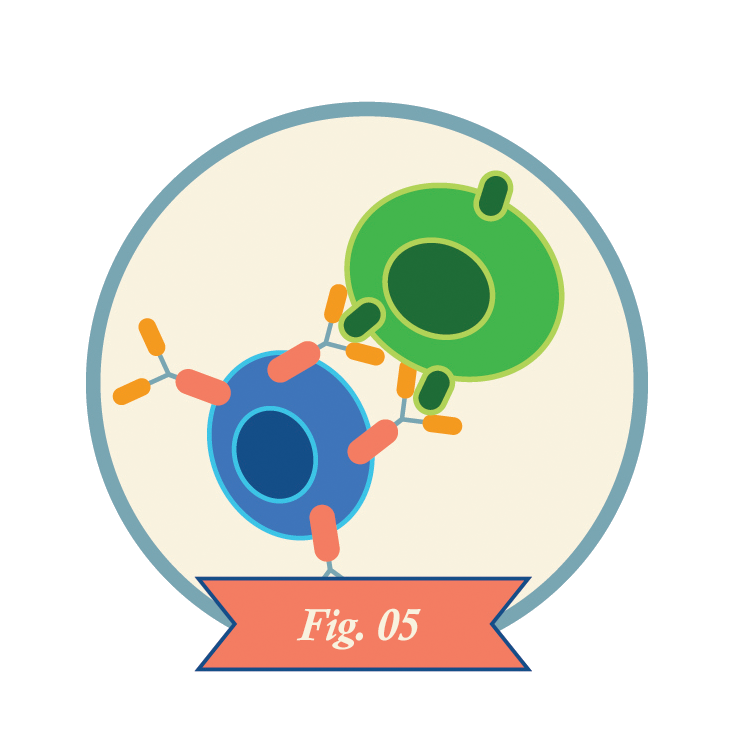
CAR-T cells recognize antigens on the surfaces of cancer cells and kill the cells. CAR-T cells may also kill healthy cells that express the same antigens.
It’s still unclear what role Hendrix’s CAR-T cell infusion prior to her surgery played in her treatment’s success. She has had no evidence of disease since her surgery in 2008, but the clinical trial she participated in was only analyzing safety and how long CAR-T cells remained in the body. Ahmed published a report in the Journal of Clinical Oncology in 2015 that showed that CAR-T cells targeting HER2-positive cancer cells can persist for six weeks in patients without causing evident toxicities. In March 2019, Ahmed’s colleague Shoba Navai, a pediatric oncologist also at Baylor, presented research at the American Association for Cancer Research (AACR) Annual Meeting showing that six out of 10 patients with HER2-positive metastatic sarcoma who were infused with HER-2-directed CAR-T cells survived for at least a year. (The AACR publishes Cancer Today.) One patient with osteosarcoma with metastasis to the lung had no evidence of disease for 32 months.
Whatever role the experimental CAR-T cell therapy played in her remission, Hendrix went on to become a pediatric nurse and got married in March 2019. She still sends blood samples back to Ahmed every year as part of ongoing efforts to monitor for any adverse effects from the genetically engineered cells.
Brown, Ahmed and Specht remain cautiously optimistic that the science of CAR-T cell therapy will continue to advance, so that one day it will be a viable treatment option for many cancer types. For now, the jury is out.
“The field of CAR-T and other cell therapies is really expansive and evolving very quickly,” Brown says. “We need to establish safety, but soon after, I expect to see a lot of interesting trials will move quickly to [begin to] establish efficacy. I think we’re right at the cusp of a very exciting time.”
Cancer Today magazine is free to cancer patients, survivors and caregivers who live in the U.S. Subscribe here to receive four issues per year.